No edit summary |
m (Reverted edits by 124.124.51.185 (talk | block) to last version by Dr Joe Kiff) |
||
| (10 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{ClinPsy}} |
{{ClinPsy}} |
||
| + | {{PsyPerspective}} |
||
| + | {{Boxtop}} |
||
| − | {{DiseaseDisorder infobox | |
||
| + | {{Infobox_Disease | |
||
| − | Name = Parkinson's disease | |
||
| − | + | Name = Parkinson's disease | |
|
| − | + | DiseasesDB = 9651 | |
|
| + | ICD10 = {{ICD10|G|20||g|20}}, {{ICD10|F|02|3|f|00}} | |
||
| + | ICD9 = {{ICD9|332}} | |
||
| + | ICDO = | |
||
| + | OMIM = | |
||
| + | MedlinePlus = 000755 | |
||
| + | eMedicineSubj = neuro | |
||
| + | eMedicineTopic = 304 | |
||
| + | eMedicine_mult = {{eMedicine2|neuro|635}} in young<br>{{eMedicine2|pmr|99}} rehab | |
||
| + | Image = Sir William Richard Gowers Parkinson Disease sketch 1886.jpg | |
||
| + | Caption = Illustration of the Parkinson disease by Sir William Richard Gowers from ''A Manual of Diseases of the Nervous System'' in 1886 | |
||
}} |
}} |
||
| + | {{Boxbottom}} |
||
| + | '''Parkinson's disease''' or '''PD''' (also known as '''Parkinson's syndrome''' or '''paralysis agitans''' ) is a neurodegenerative disorder of the [[central nervous system]] that often impairs the sufferer's [[motor skill]]s, speech, and other functions.<ref name=Jankovic2008/> |
||
| + | Parkinson's disease belongs to a group of conditions called [[movement disorder]]s. It is characterized by muscle rigidity, tremor, a slowing of physical movement ([[bradykinesia]]) and, in extreme cases, a loss of physical movement ([[akinesia]]). The primary symptoms are the results of decreased stimulation of the [[motor cortex]] by the [[basal ganglia]], normally caused by the insufficient formation and action of [[dopamine]], which is produced in the [[dopaminergic neuron]]s of the brain. Secondary symptoms may include high level cognitive dysfunction and subtle language problems. PD is both [[Chronic (medicine)|chronic]] and progressive. |
||
| − | '''Parkinson's disease''' (paralysis agitans or PD) is a [[neurodegenerative disease]] of the [[substantia nigra]], an area in the [[basal ganglia]] of the [[brain]]. The disease was first recognised and its symptoms documented in [[1817]] in ''An Essay on the Shaking Palsy'' by the British physician Dr [[James Parkinson]]; the associated [[biochemical]] changes in the [[brain]] of [[patient]]s were identified in the [[1960s]]. Some [[gene]] defects associated with the disease were identified only recently; others remain unknown. |
||
| + | PD is the most common cause of chronic progressive [[parkinsonism]], a term which refers to the syndrome of tremor, rigidity, bradykinesia and postural instability. PD is also called "primary parkinsonism" or "[[idiopathic]] PD" (classically meaning having no known cause although this term is not strictly true in light of the plethora of newly discovered genetic mutations). While many forms of parkinsonism are "idiopathic", "secondary" cases may result from toxicity most notably of drugs, head trauma, or other medical disorders. The disease is named after English physician [[James Parkinson]], who made a detailed description of the disease in his essay: "An Essay on the Shaking Palsy" (1817). |
||
| − | The disease involves a progressive disorder of the [[extrapyramidal system]], which controls and adjusts communication between [[neuron]]s in the brain and [[muscle]]s in the [[human body]]. It also commonly involves [[depression (mood)|depression]] and disturbances of sensory systems. |
||
| + | ==Classification== |
||
| − | Parkinson's disease is widespread, with a prevalence estimated between 100 and 250 cases per 100,000 in North America; globally prevalence estimates range from a low of 15 per 100,000 in China to a high of 657 per 100,000 in Argentina. Because prevalence rates can be affected by socio-economically driven differences in survival, incidence is a more sensitive indicator: rates have ranged from 1.5 per 100,000 in China to a high of 14.8 per 100,000 in Finland. [BC Medical Journal Volume 43, Number 3, April 2001, 133-137 |
||
| − | Epidemiology of Parkinson’s disease |
||
| − | Benjamin C.L. Lai, MD, MSc, and Joseph K.C. Tsui, MD, FRCP(UK), FRCPC] |
||
| + | "Parkinson's disease" is the synonym of "primary parkinsonism", i.e. isolated parkinsonism due to a neurodegenerative process without any secondary systemic cause. In some cases, it would be inaccurate to say that the cause is "unknown", because a small proportion is caused by genetic mutations. It is possible for a patient to be initially diagnosed with Parkinson's disease but then to develop additional features, requiring revision of the diagnosis.<ref name=nice35/> |
||
| − | About 2% of the population develops the disease some time during life, though the mean age at onset is 58-60. Symptoms usually begin in the upper extremities, and are usually unilateral (one-sided) or asymmetrical at onset. |
||
| + | There are other disorders that are called ''[[Parkinson plus syndrome|Parkinson-plus diseases]]''. These include: [[multiple system atrophy]] (MSA), [[progressive supranuclear palsy]] (PSP) and [[corticobasal degeneration]] (CBD). Some include [[dementia with Lewy bodies]] (DLB) — while idiopathic Parkinson's disease patients also have [[Lewy body|Lewy bodies]] in their brain tissue, the distribution is denser and more widespread in DLB. Even so, the relationship between Parkinson disease, Parkinson disease with dementia (PDD), and dementia with Lewy bodies (DLB) might be most accurately conceptualized as a spectrum, with a discrete area of overlap between each of the three disorders. The natural history and role of Lewy bodies is little understood. |
||
| − | ==Symptoms== |
||
| + | These Parkinson-plus diseases may progress more quickly than typical idiopathic Parkinson disease. If cognitive dysfunction occurs before or very early in the course of the movement disorder then DLBD may be suspected. Early postural instability with minimal tremor especially in the context of ophthalmoparesis should suggest PSP. Early autonomic dysfunction including erectile dysfunction and syncope may suggest MSA. The presence of extreme asymmetry with patchy cortical cognitive defects such as dysphasia and apraxias especially with "alien limb" phenomena should suggest CBD. |
||
| − | Parkinson disease affects movement (motor symptoms). Typical other symptoms include disorders of mood, behavior, thinking, and sensation (non-motor symptoms). Individual patients' symptoms may be quite dissimilar; progression is also distinctly individual, presumably because the pattern of brain cell pathology is individual. |
||
| + | The usual anti-Parkinson's medications are typically either less effective or not effective at all in controlling symptoms; patients may be exquisitely sensitive to neuroleptic medications like [[haloperidol]]. Additionally, the [[Acetylcholinesterase inhibitor|cholinesterase inhibiting]] medications have shown preliminary efficacy in treating the cognitive, psychiatric, and behavioral aspects of the disease, so correct differential diagnosis is important. |
||
| − | ===Motor symptoms=== |
||
| + | [[Essential tremor]] may be mistaken for Parkinson's disease but lacks all other features besides tremor, and has particular characteristics distinguishing it from Parkinson's, such as improvement with [[beta blocker]]s and [[alcoholic beverage]]s.<ref name=Jankovic2008/> |
||
| − | The [[cardinal symptom]]s are: |
||
| − | *'''[[tremor]]''': 4-7[[Hertz|Hz]] tremor, maximal when the limb is at rest and decreased with voluntary movement. It is typically unilateral at onset. This is the most apparent and well-known symptom. However, an estimated 30% of patients have little perceptible tremor; these are classified as akinetic-rigid. |
||
| − | *'''[[rigidity]]''': stiffness; increased muscle tone. In combination with a resting tremor, this produces a ratchety, "[[cogwheel]]" rigidity when the limb is passively moved. |
||
| − | *'''[[bradykinesia]]/[[akinesia]]''': respectively, slowness or absence of movement. Rapid, repetitive movements produce a [[rhythm|dysrhythmic]] and decremental loss of [[amplitude]]. |
||
| − | *'''[[postural instability]]''': failure of postural [[reflexes]], which leads to impaired balance and falls. |
||
| − | (The [[mnemonic]] '''''TRAP''''' ('''T'''remor; '''R'''igidity; '''A'''kinesia/bradykinesia; '''P'''ostural instability) can be used to remember these symptoms.) |
||
| + | [[Wilson's disease]] (hereditary copper accumulation) may present with parkinsonian features; young patients presenting with parkinsonism or any other movement disorder are frequently screened for this rare condition, because it may respond to medical treatment. Typical tests are [[liver enzymes|liver function]], slit lamp examination for [[Kayser-Fleischer ring]]s, and serum [[ceruloplasmin]] levels. |
||
| − | Other motor symptoms include: |
||
| + | ==Signs and symptoms== |
||
| − | *[[Gait]] and Posture Disturbances: |
||
| + | Parkinson disease affects movement (motor symptoms). Other typical symptoms include disorders of mood, behaviour, thinking, and sensation (non-motor symptoms). Patients' individual symptoms may be quite dissimilar and progression of the disease is also distinctly individual. |
||
| − | **Shuffling: gait is characterized by short steps, with feet barely leaving the ground, producing an audible shuffling noise. Small obstacles tend to trip the patient |
||
| + | |||
| − | **Decreased arm swing: a form of bradykinesia |
||
| + | ===Motor symptoms=== |
||
| + | The [[cardinal symptom]]s are (mnemonic "TRAP"):<ref name=Jankovic2008>{{cite journal |author=Jankovic J |title=Parkinson's disease: clinical features and diagnosis |journal=J. Neurol. Neurosurg. Psychiatr. |volume=79 |issue=4 |pages=368–76 |year=2008 |month=April |pmid=18344392 |doi=10.1136/jnnp.2007.131045}}</ref> |
||
| + | *''[[Tremor]]'': normally 4-6 [[Hertz|Hz]] tremor, maximal when the limb is at rest, and decreased with voluntary movement. It is typically unilateral at onset. This is the most apparent and well-known symptom, though an estimated 30% of patients have little perceptible tremor; these are classified as akinetic-rigid. |
||
| + | *''[[Spasticity|Rigidity]]'': stiffness; increased muscle tone. In combination with a resting tremor, this produces a ratchety, "cogwheel" rigidity when the limb is passively moved. |
||
| + | *''[[bradykinesia]]/[[Akinesia]]'': respectively, slowness or absence of movement. Rapid, repetitive movements produce a [[rhythm|dysrhythmic]] and decremental loss of [[amplitude]]. |
||
| + | *''[[Postural instability]]'': failure of postural [[reflexes]], which leads to impaired balance and falls. |
||
| + | |||
| + | Other motor symptoms include: |
||
| + | *[[Gait]] and posture disturbances: |
||
| + | **Shuffling: gait is characterized by short steps, with feet barely leaving the ground, producing an audible shuffling noise. Small obstacles tend to cause the patient to trip. |
||
| + | **Decreased arm-swing. |
||
**Turning "en bloc": rather than the usual twisting of the neck and trunk and pivoting on the toes, PD patients keep their neck and trunk rigid, requiring multiple small steps to accomplish a turn. |
**Turning "en bloc": rather than the usual twisting of the neck and trunk and pivoting on the toes, PD patients keep their neck and trunk rigid, requiring multiple small steps to accomplish a turn. |
||
| − | **Stooped, forward-flexed posture. In severe forms, the head and upper shoulders may be bent at a [[right angle]] relative to the trunk ( |
+ | **Stooped, forward-flexed posture. In severe forms, the head and upper shoulders may be bent at a [[right angle]] relative to the trunk (camptocormia). <ref>{{cite journal |author=Lepoutre A, Devos D, Blanchard-Dauphin A, ''et al'' |title=A specific clinical pattern of camptocormia in Parkinson's disease |journal=J. Neurol. Neurosurg. Psychiatr. |volume=77 |issue=11 |pages=1229–34 |year=2006 |pmid=16735399 |doi=10.1136/jnnp.2005.083998}}</ref> |
**Festination: a combination of stooped posture, imbalance, and short steps. It leads to a gait that gets progressively faster and faster, often ending in a fall. |
**Festination: a combination of stooped posture, imbalance, and short steps. It leads to a gait that gets progressively faster and faster, often ending in a fall. |
||
| − | **Gait freezing: |
+ | **Gait freezing: "freezing" is a manifestation of akinesia (an inability to move). Gait freezing is characterized by an inability to move the feet which may worsen in tight, cluttered spaces or when attempting to initiate gait. |
| − | **[[Dystonia]]: abnormal, sustained, painful twisting muscle contractions, |
+ | **[[Dystonia]] (in about 20% of cases): abnormal, sustained, painful twisting muscle contractions, often affecting the foot and ankle (mainly toe flexion and foot inversion) which often interferes with gait. |
| − | *Speech and |
+ | *Speech and swallowing disturbances. |
| − | **Hypophonia: soft speech. |
+ | **Hypophonia: soft speech. Speech quality tends to be soft, hoarse, and monotonous. Some people with Parkinson's disease claim that their tongue is "heavy" or have [[cluttered speech]].<ref>{{cite book | first=Michael | middle=J | last=Fox | title= Lucky Man: A Memoir | location = | publisher= Hyperion | isbn=0786888741 | pages=214 | year = 2003}}</ref> |
| + | **Monotonic speech. |
||
**Festinating speech: excessively rapid, soft, poorly-intelligible speech. |
**Festinating speech: excessively rapid, soft, poorly-intelligible speech. |
||
**[[Drooling]]: most likely caused by a weak, infrequent swallow and stooped posture. |
**[[Drooling]]: most likely caused by a weak, infrequent swallow and stooped posture. |
||
| + | **[[Dysphagia]]: impaired ability to swallow. Can lead to [[Pulmonary aspiration|aspiration]], [[pneumonia]]. |
||
| − | **(Non-motor causes of speech/language disturbance in both expressive and receptive language: these include decreased verbal fluency and cognitive disturbance especially related to comprehension of emotional content of speech and of facial expression[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8954247&query_hl=8&itool=pubmed_docsum]) |
||
| − | **[[Dysphagia]]: impaired ability to swallow. Can lead to [[aspiration]], [[pneumonia]], and death. |
||
*Other motor symptoms: |
*Other motor symptoms: |
||
| − | **[[fatigue]] (up to 50% of cases); |
+ | **[[fatigue (physical)|Fatigue]] (up to 50% of cases); |
| − | ** |
+ | **Masked faces (a mask-like face also known as [[hypomimia]]), with infrequent [[blinking]];<ref>{{cite journal |author=Deuschl G, Goddemeier C |title=Spontaneous and reflex activity of facial muscles in dystonia, Parkinson's disease, and in normal subjects |journal=J. Neurol. Neurosurg. Psychiatr. |volume=64 |issue=3 |pages=320–4 |year=1998 |pmid=9527141 |url=http://jnnp.bmjjournals.com/cgi/content/full/64/3/320}}</ref> |
| − | ** |
+ | **Difficulty rolling in bed or rising from a seated position; |
| − | **[[micrographia]] (small, cramped handwriting); |
+ | **[[micrographia (handwriting)|Micrographia]] (small, cramped handwriting); |
| − | ** |
+ | **Impaired fine motor dexterity and [[motor coordination]]; |
| − | ** |
+ | **Impaired gross motor coordination; |
| + | **[[Akathisia]], the inability to sit still. |
||
| − | **"Poverty of movement: overall loss of accessory movements, such as decreased arm swing when walking, as well as spontaneous movement. |
||
| − | === |
+ | ===Neuropsychiatric=== |
| + | PD causes cognitive and mood disturbances, being in many cases related. |
||
| + | Estimated prevalence rates of depression vary widely according to the population sampled and methodology used. Reviews of [[clinical depression|depression]] estimate its occurrence in anywhere from 20-80% of cases.<ref>{{cite journal | author = Lieberman A | title = Depression in Parkinson's disease -- a review | journal = Acta Neurol Scand | volume = 113 | issue = 1 | pages = 1–8 | year = 2006|pmid = 16367891 | doi = 10.1111/j.1600-0404.2006.00536.x}}</ref> Estimates from community samples tend to find lower rates than from specialist centres. Most studies use self-report questionnaires such as the [[Beck Depression Inventory]], which may overinflate scores due to physical symptoms. Studies using diagnostic interviews by trained psychiatrists also report lower rates of depression. More generally, there is an increased risk for any individual with depression to go on to develop Parkinson's disease at a later date.<ref>{{cite journal | author = Ishihara L, Brayne C | title = A systematic review of depression and mental illness preceding Parkinson's disease | journal = Acta Neurol Scand | volume = 113 | issue = 4 | pages = 211–20 | year = 2006 | pmid = 16542159 | doi = 10.1111/j.1600-0404.2006.00579.x}}</ref> Seventy percent of individuals with Parkinson's disease diagnosed with pre-existing depression go on to develop anxiety. Ninety percent of Parkinson's disease patients with pre-existing anxiety subsequently develop depression; [[apathy]] or [[abulia]]. |
||
| − | Mood Disturbances: |
||
| − | *[[clinical depression|depression]]: occurs in 40-70% of cases; 20% of depression cases are major depressive disorder; severity and persistance of depression is positively associated with executive dysfunction and dementia; |
||
| − | *[[anxiety]] or [[panic attacks]]<br>Note: 70% of individuals with parkinson's disease diagnosed with pre-existing depression go on to develop anxiety; 90% of Parkinson's disease patients with pre-existing anxiety subsequently develop depression); |
||
| − | *[[apathy]] or [[abulia]]: abulia translates from Greek as the absence or negative of will; apathy is an absence of feeling or desire |
||
| − | + | Cognitive disturbances include: |
|
| − | *[[ |
+ | *[[Slowed reaction time]]; both voluntary and involuntary motor responses are significantly slowed. |
| − | *[[ |
+ | *[[Executive dysfunction]], characterized by difficulties in: differential allocation of attention, impulse control, set shifting, prioritizing, evaluating the salience of ambient data, interpreting social cues, and subjective time awareness. This complex is present to some degree in most Parkinson's patients; it may progress to: |
| − | *[[ |
+ | *[[Dementia]]: a later development in approximately 20-40% of all patients, typically starting with slowing of thought and progressing to difficulties with abstract thought, memory, and behavioral regulation. [[Hallucinations]], [[delusions]] and [[paranoia]] may develop. |
| − | *[[memory loss]]; procedural memory is more impaired than declarative memory. Prompting elicits improved recall. |
+ | *Short term [[memory loss]]; [[procedural memory]] is more impaired than [[declarative memory]]. Prompting elicits improved recall. |
| + | *Non-motor causes of speech/language disturbance in both expressive and receptive language: these include decreased verbal fluency and cognitive disturbance especially related to comprehension of emotional content of speech and of facial expression.<ref>{{cite journal | author = Pell M | title = On the receptive prosodic loss in Parkinson's disease | journal = Cortex | volume = 32 | issue = 4 | pages = 693–704 | year = 1996 | pmid = 8954247}}</ref> |
||
| + | *Medication effects: some of the above cognitive disturbances are improved by dopaminergic medications, while others are actually worsened.<ref>{{cite journal |author=Frank MJ |title=Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism |journal=Journal of cognitive neuroscience |volume=17 |issue=1 |pages=51–72 |year=2005 |pmid=15701239 |doi=10.1162/0898929052880093}}</ref> |
||
| + | ===Sleep=== |
||
| − | [[Sleep Disturbances]]: |
||
| − | *Excessive daytime somnolence |
+ | *Excessive daytime [[somnolence]] |
| − | *Initial, intermediate, and terminal insomnia |
+ | *Initial, intermediate, and terminal [[insomnia]] |
| − | *Disturbances in REM sleep: disturbingly vivid dreams, and REM Sleep Disorder, characterized by acting out of dream content |
+ | *Disturbances in [[Rapid eye movement sleep|REM]] sleep: disturbingly vivid dreams, and REM Sleep Disorder, characterized by acting out of dream content — can occur years prior to diagnosis |
| + | ===Perception=== |
||
| − | [[Sensation]] Disturbances: |
||
| − | * |
+ | *Impaired visual [[contrast sensitivity]], spatial reasoning, [[color|colour]] discrimination, [[convergence insufficiency]] (characterized by [[double vision]]) and [[oculomotor control]] |
| − | *[[ |
+ | *[[Dizziness]] and fainting; usually attributable [[orthostatic hypotension]], a failure of the [[autonomic nervous system]] to adjust blood pressure in response to changes in body position |
| − | * |
+ | *Impaired [[proprioception]] (the awareness of bodily position in three-dimensional space) |
| − | *loss of sense of [[smell]] ([[anosmia]]) |
+ | *Reduction or loss of sense of [[olfaction|smell]] ([[hyposmia]] or [[anosmia]]) - can occur years prior to diagnosis |
*[[pain]]: neuropathic, muscle, joints, and tendons, attributable to tension, dystonia, rigidity, joint stiffness, and injuries associated with attempts at accommodation |
*[[pain]]: neuropathic, muscle, joints, and tendons, attributable to tension, dystonia, rigidity, joint stiffness, and injuries associated with attempts at accommodation |
||
| + | ===Autonomic=== |
||
| − | [[autonomic nervous system|Autonomic]] disturbances: |
||
| + | *Oily skin and [[seborrheic dermatitis]]<ref>{{cite journal |author=Gupta A, Bluhm R |title=Seborrheic dermatitis |journal=Journal of the European Academy of Dermatology and Venereology : JEADV |volume=18 |issue=1 |pages=13–26; quiz 19–20 |year=2004 |pmid=14678527 |doi=10.1111/j.1468-3083.2004.00693.x}}</ref> |
||
| − | *[[oily skin]] and [[seborrheic dermatitis]]; |
||
| − | *[[ |
+ | *[[Urinary incontinence]], typically in later disease progression |
| + | *Nocturia (getting up in the night to pass urine) — up to 60% of cases |
||
| − | *[[constipation]] and [[gastric]]dysmotility: severe enough to endanger comfort and even health |
||
| + | *[[Constipation]] and [[gastric]] dysmotility that is severe enough to endanger comfort and even health |
||
| − | *[[altered sexual function]]: characterized by profound impairment of sexual arousal, behavior, orgasm, and drive is found in mid and late parkinson disease. Current data addresses male sexual function almost exclusively. |
||
| + | * Altered sexual function: characterized by profound impairment of sexual arousal, behavior, orgasm, and drive is found in mid and late Parkinson disease. Current data addresses male sexual function almost exclusively. |
||
| + | *[[Weight loss]], which is significant over a period of ten years. |
||
| − | == |
+ | ==Causes== |
| + | Most people with Parkinson's disease are described as having [[idiopathic]] Parkinson's disease (having no specific cause). There are far less common causes of Parkinson's disease including genetic, toxins, head trauma, cerebral [[Hypoxia (medical)|anoxia]], and drug-induced Parkinson's disease. |
||
| − | Symptoms usually only begin to appear after about 80% of the [[Dopamine|dopamine]] in the brain has been lost. More recent data based on [[Positron emission tomography|PET scans]] suggests that symptoms may occur when 50-60% of dopaminergic neurons are lost. The level of [[Dopamine|dopamine]] will continue to fall slowly over time, with an attendant worsening of symptoms. |
||
| + | ===Genetic=== |
||
| − | It is an incapacitating disease, disturbing important human functions and ultimately substantially reducing quality of life. As in many [[neurologic diseases]], [[psychology|psychological]] complications are often extremely serious and require the patient's family members and relatives to pay keen attention to the emotional fragility that usually follows the emergence of the disease; indeed, the [[clinical depression|depression]] which often results is seen by many as one of the worst aspects of the disease. |
||
| + | In recent years, a number of specific genetic mutations causing Parkinson's disease have been discovered, including in certain populations ([[Contursi]], Italy). These account for a small minority of cases of Parkinson's disease. Someone who has Parkinson's disease is more likely to have relatives that also have Parkinson's disease.However, this does not mean that the disorder has been passed on genetically. |
||
| + | ===Toxins=== |
||
| − | Fairly effective medication for the movement difficulties of Parkinson disease have been available for some time, but the neuropsychiatric aspects of the disease, especially depression and anxiety, are more recently characterized, less well understood, and often less adequately treated. As patients become more disabled, they become more dependent on care from others to perform all manner of tasks, from eating and bathing to monitoring and taking medication. Helping individuals with chronic disability and psychiatric comorbidity to maintain purposeful engagement with life takes a physical and emotional toll on caregivers, who may consequently experience illness and depression themselves. |
||
| + | One theory holds that the disease may result in many or even most cases from the combination of a genetically determined vulnerability to environmental [[toxin]]s along with exposure to those toxins.<ref>{{cite journal |author=Di Monte DA, Lavasani M, Manning-Bog AB |title=Environmental factors in Parkinson's disease |journal=Neurotoxicology |volume=23 |issue=4-5 |pages=487–502 |year=2002 |pmid=12428721 |doi=}}</ref> This hypothesis is consistent with the fact that Parkinson's disease is not distributed homogeneously throughout the population; its incidence varies geographically. However, it is not consistent with the fact that the first appearance of the syndrome predates the first synthesis of the compounds often attributed to causing Parkinson's disease. The toxins most strongly suspected at present are certain [[pesticide]]s and transition-series metals such as [[Manganism|manganese]] or iron, especially those that generate [[reactive oxygen species]],<ref name="Jenner1998">{{cite journal |author=Jenner P |title=Oxidative mechanisms in nigral cell death in Parkinson's disease |journal=Mov. Disord. |volume=13 Suppl 1 |issue= |pages=24–34 |year=1998 |pmid=9613715 |doi=}}</ref><ref>{{cite journal |author=Chiueh CC, Andoh T, Lai AR, Lai E, Krishna G |title=Neuroprotective strategies in Parkinson's disease: protection against progressive nigral damage induced by free radicals |journal=Neurotoxicity research |volume=2 |issue=2-3 |pages=293–310 |year=2000 |pmid=16787846 |doi=}}</ref> |
||
| + | and/or bind to [[neuromelanin]], as originally suggested by G.C. Cotzias.<ref>{{cite journal | author = Cotzias G | title = Manganese, melanins and the extrapyramidal system | journal = J Neurosurg | volume = 24 | issue = 1 | pages = Suppl:170–80 | year = 1966 | pmid = 4955707}}</ref><ref>{{cite journal | author = Barbeau A | title = Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) | journal = Neurotoxicology | volume = 5 | issue = 1 | pages = 13–35 | year = 1984 | pmid = 6538948}}</ref> In the Cancer Prevention Study II Nutrition Cohort, a longitudinal investigation, individuals who were exposed to pesticides had a 70% higher incidence of PD than individuals who were not exposed.<ref>{{cite journal | author = Ascherio A, Chen H, Weisskopf M, ''et al'' | title = Pesticide exposure and risk for Parkinson's disease | journal = Ann Neurol | volume = 60 | issue = 2 | pages = 197–203 | year = 2006 | pmid = 16802290 | doi = 10.1002/ana.20904}}</ref> |
||
| + | The tragedy of a group of drug addicts in California in the early 1980s who consumed a contaminated and illicitly produced batch of the synthetic opiate [[MPPP]] brought to light [[MPTP]] (pro-toxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyidine) as a specific cause of Parkinson symptoms. This made it possible to develop the first animal model for Parkinson's. MPTP's toxicity likely comes from the generation of [[reactive oxygen species]] through tyrosine hydroxylation.<ref>{{cite journal | author = Chiueh C, Wu R, Mohanakumar K, Sternberger L, Krishna G, Obata T, Murphy D | title = ''In vivo'' generation of hydroxyl radicals and MPTP-induced dopaminergic toxicity in the basal ganglia | journal = Ann N Y Acad Sci | volume = 738 | issue = | pages = 25–36 | year = 1994 |pmid = 7832434}}</ref> [[The Case of the Frozen Addicts]] by [[J. William Langston]] (Vintage, New York, June 25, 1996) documents this tragedy and describes the first attempts at fetal brain tissue transplants to treat PD. |
||
| − | Cases of PD are reported at all ages, though it is quite rare in people younger than 30 and the average age at which symptoms begin is 58-60; the risk of developing it substantially increases with age. It occurs in all parts of the world, but appears to be more common in people of European ancestry than in those of African ancestry. Those of East Asian ancestry have an intermediate risk. It is more common in rural than urban areas and men are affected slightly more often than women. |
||
| + | Other toxin-based models employ PCBs,<ref>{{cite news |
||
| − | == Diagnosis == |
||
| + | |first=Leslie |
||
| − | ===Differential diagnosis=== |
||
| + | |last=Orr |
||
| − | The [[differential diagnosis]] for a patient presenting with Parkinsonian symptoms is: |
||
| − | + | |title=PCBs, fungicide open brain cells to Parkinson's assault |
|
| + | |date=February 10, 2005 |
||
| − | * [[Essential tremor]] (ET): tremor is typically associated with posture-holding and voluntary movement, and absent at rest. A head tremor suggests ET; a lip or chin tremor is more typical of PD. |
||
| + | |publisher=[[Medical News Today]] |
||
| − | * [[Parkinson plus syndrome]]s (see below) |
||
| + | |url=http://www.medicalnewstoday.com/medicalnews.php?newsid=19791 |
||
| − | * Secondary parkinsonism due to [[recreational drug use|drug]]s, [[toxin]]s, [[stroke]], [[head trauma]], or [[hydrocephalus]] |
||
| + | }}</ref> [[paraquat]]<ref>{{cite journal |author=Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA |title=The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein |journal=J. Biol. Chem. |volume=277 |issue=3 |pages=1641–4 |year=2002 |pmid=11707429 |doi=10.1074/jbc.C100560200 | url=http://www.jbc.org/cgi/content/full/277/3/1641}}</ref> (a herbicide) in combination with maneb (a fungicide),<ref>{{cite journal |author=Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA |title=The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease |journal=J. Neurosci. |volume=20 |issue=24 |pages=9207–14 |year=2000 |pmid=11124998 |url=http://www.jneurosci.org/cgi/content/full/20/24/9207 |
||
| + | }}</ref> [[rotenone]]<ref>{{cite journal |author=Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT |title=Chronic systemic pesticide exposure reproduces features of Parkinson's disease |journal=Nat. Neurosci. |volume=3 |issue=12 |pages=1301–6 |year=2000 |pmid=11100151 |doi=10.1038/81834}}</ref> (an insecticide), and specific organochlorine pesticides including dieldrin<ref>{{cite journal |author=Kitazawa M, Anantharam V, Kanthasamy AG |title=Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells |journal=Free Radic. Biol. Med. |volume=31 |issue=11 |pages=1473–85 |year=2001 |pmid=11728820 |url=http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T38-44HSN76-P&_coverDate=12%2F01%2F2001&_alid=373422978&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=4940&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5a104ac89bd7948e14863371142a639a |doi=10.1016/S0891-5849(01)00726-2 |
||
| + | }}</ref> and lindane.<ref>{{cite journal |author=Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D |title=Organochlorine insecticides in substantia nigra in Parkinson's disease |journal=J. Toxicol. Environ. Health Part A |volume=59 |issue=4 |pages=229–34 |year=2000 |pmid=10706031 |
||
| + | |url=http://journalsonline.tandf.co.uk/openurl.asp?genre=article&eissn=1087-2620&volume=59&issue=4&spage=229 |doi=10.1080/009841000156907 |
||
| + | }}</ref> Rotenone is an inhibitor of complex 1 of the electron transport chain. It easily crosses membranes due to its extremely hydrophobic properties. Rotenone, therefore, does not rely on the dopamine transporter to enter into the cytoplasm. Numerous studies have found an increase in PD in persons who consume rural well water; researchers theorize that water consumption is a proxy measure of pesticide exposure. In agreement with this hypothesis are studies which have found a dose-dependent increase in PD in persons exposed to agricultural chemicals. |
||
| + | ===Head trauma=== |
||
| − | Parkinson's tremors differ from essential tremors in that the latter are posture or action tremors, have bilateral tremors involving the hands, head and voice, and are alcohol responsive. |
||
| + | Past episodes of head trauma are reported more frequently by individuals with Parkinson's disease than by others in the population.<ref name=Bower>{{cite journal |author=Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA |title=Head trauma preceding PD: a case-control study |journal=Neurology |volume=60 |issue=10 |pages=1610–5 |year=2003 |pmid=12771250 | url=http://www.neurology.org/cgi/content/abstract/60/10/1610 |format=abstract page}}</ref><ref>{{cite journal |author=Stern M, Dulaney E, Gruber SB, ''et al'' |title=The epidemiology of Parkinson's disease. A case-control study of young-onset and old-onset patients |journal=Arch. Neurol. |volume=48 |issue=9 |pages=903–7 |year=1991 |pmid=1953412 url=http://archneur.ama-assn.org/cgi/content/abstract/48/9/903}}</ref><ref name="Uryu2003">{{cite journal |author=Uryu K, Giasson BI, Longhi L, ''et al'' |title=Age-dependent synuclein pathology following traumatic brain injury in mice |journal=Exp. Neurol. |volume=184 |issue=1 |pages=214–24 |year=2003 |pmid=14637093 |doi=}}</ref> |
||
| − | In contrast, Parkinson's tremors are rest tremors, and usually start unilaterally. |
||
| + | A recent methodologically strong retrospective study<ref name=Bower/> found that those who have experienced a head injury are four times more likely to develop Parkinson’s disease than those who have never suffered a head injury. The risk of developing Parkinson’s increases eightfold for patients who have had head trauma requiring hospitalization, and it increases 11-fold for patients who had experienced severe head injury. The authors comment that since head trauma is a rare event, the contribution to PD incidence is slight. They express further concern that their results may be biased by recall, i.e., the PD patients because they reflect upon the causes of their illness, may remember head trauma better than the non-ill control subjects. These limitations were overcome recently by Tanner and colleagues,<ref>{{cite journal |author=Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW |title=Head injury and Parkinson's disease risk in twins |journal=Ann. Neurol. |volume=60 |issue=1 |pages=65–72 |year=2006 |pmid=16718702 |doi=10.1002/ana.20882}}</ref> who found a similar risk of 3.8, with increasing risk associated with more severe injury and hospitalization. However, whether the head trauma actually contributed to Parkinson's disease development or the early symptoms of clumsiness associated with Parkinson's causes individuals to have more head trauma is still unknown. |
||
| − | == |
+ | ==Pathophysiology== |
| + | [[Image:DA-loops in PD.jpg|thumb|250px|Dopaminergic pathways of the human brain in normal condition (left) and Parkinson's disease (right). Red Arrows indicate suppression of the target, blue arrows indicate stimulation of target structure.]] |
||
| − | [[SPECT]] with ([123I][[FP-CIT]]) or [[positron emission tomography|PET]] with <sup>18</sup>F-fluorodopa are the two [[medical imaging|imaging]] modalities used to assess dopamine transporter density and the integrity of nigrostriatal pathways in the central nervous system. Currently (2005) FP-CIT is widely used in Europe for the diagnostic workup of Clinically Uncertain Parkinsonian Syndromes; although it is not available in the United States. |
||
| + | The symptoms of Parkinson's disease result from the loss of pigmented [[dopamine]]-secreting (dopaminergic) cells in the [[pars compacta]] region of the [[substantia nigra]] (literally "black substance"). These neurons project to the [[striatum]] and their loss leads to alterations in the activity of the neural circuits within the basal ganglia that regulate movement, in essence an inhibition of the [[direct pathway]] and excitation of the [[indirect pathway]]. |
||
| + | The direct pathway facilitates movement and the indirect pathway inhibits movement, thus the loss of these cells leads to a hypokinetic movement disorder. The lack of [[dopamine]] results in increased inhibition of the ventral anterior nucleus of the thalamus, which sends excitatory projections to the [[motor cortex]], thus leading to [[hypokinesia]]. |
||
| − | ==Related diseases== |
||
| − | ===Parkinson-Plus diseases=== |
||
| − | There are other disorders that are called '''[[Parkinson plus syndrome|Parkinson-Plus diseases]]'''. These include: |
||
| + | There are four major dopamine pathways in the brain; the nigrostriatal pathway, referred to above, mediates movement and is the most conspicuously affected in early Parkinson's disease. The other pathways are the mesocortical, the mesolimbic, and the tuberoinfundibular. Disruption of dopamine along the non-striatal pathways likely explains much of the neuropsychiatric pathology associated with Parkinson's disease. |
||
| − | * '''[[Multiple System Atrophy]] (MSA)''' |
||
| − | ** '''[[Shy-Drager Syndrome]] (SDS)''' |
||
| − | ** '''Striatonigral degeneration (SND)''' |
||
| − | ** '''Olivopontocerebellar Atrophy (OPCA)''' |
||
| − | * '''Progressive Supranuclear Palsy (PSP)''' |
||
| − | * '''Corticobasal Degeneration (CBD)''' |
||
| + | The mechanism by which the brain cells in Parkinson's are lost may consist of an abnormal accumulation of the protein [[alpha-synuclein]] bound to ubiquitin in the damaged cells. The [[alpha-synuclein]]-ubiquitin complex cannot be directed to the proteosome. This [[protein]] accumulation forms proteinaceous cytoplasmic inclusions called [[Lewy bodies]]. The latest research on pathogenesis of disease has shown that the death of dopaminergic neurons by alpha-synuclein is due to a defect in the machinery that transports proteins between two major cellular organelles — the endoplasmic reticulum (ER) and the Golgi apparatus. Certain proteins like Rab1 may reverse this defect caused by alpha-synuclein in animal models.<ref>"Parkinson's Disease Mechanism Discovered," [http://www.hhmi.org/news/lindquist20060622.html HHMI Research News] June 22, 2006.</ref> |
||
| − | Some people include Dementia with Lewy Bodies (DLB) as one of the 'Parkinson-Plus' syndromes. Although Idiopathic Parkinson Disease patients also have Lewy bodies in their brain tissue, the distibution is denser and more widespread in DLB. Even so, the relationship between Parkinson disease, Parkinson disease with Demnentia (PDD) and Dementia with Lewy Bodies (DLB) might be most accurately conceptualized as an spectrum, with a discrete area of overlap between each of the three disorders. The natural history and role of Lewy bodies is very little understood. |
||
| + | Excessive accumulations of iron, which are toxic to nerve cells, are also typically observed in conjunction with the protein inclusions. Iron and other [[transition metals]] such as copper bind to [[neuromelanin]] in the affected neurons of the [[substantia nigra]]. [[Neuromelanin]] may be acting as a protective agent. The most likely mechanism is generation of [[reactive oxygen species]].<ref name="Jenner1998">{{cite journal | author = Jenner P | title = Oxidative mechanisms in nigral cell death in Parkinson's disease | journal = Mov Disord | volume = 13 Suppl 1 | issue = | pages = 24–34 | year =1998 | pmid = 9613715}}</ref> Iron also induces aggregation of synuclein by oxidative mechanisms.<ref>{{cite journal | author = Kaur D, Andersen J | title = Ironing out Parkinson's disease: is therapeutic treatment with iron chelators a real possibility? | journal = Aging Cell | volume = 1 | issue = 1 | pages = 17–21 | year = 2002 | pmid = 12882349 | url=http://www.blackwell-synergy.com/doi/pdf/10.1046/j.1474-9728.2002.00001.x | format=PDF | doi = 10.1046/j.1474-9728.2002.00001.x}}</ref> Similarly, dopamine and the byproducts of dopamine production enhance alpha-synuclein aggregation. The precise mechanism whereby such aggregates of alpha-synuclein damage the cells is not known. The aggregates may be merely a normal reaction by the cells as part of their effort to correct a different, as-yet unknown, insult. Based on this mechanistic hypothesis, a [[Genetically modified organism|transgenic mouse model]] of Parkinson's has been generated by introduction of human wild-type alpha-synuclein into the mouse genome under control of the [[Platelet-derived growth factor|platelet-derived-growth factor]]-β promoter.<ref>{{cite journal |author=Masliah E, Rockenstein E, Veinbergs I, ''et al'' |title=Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders |journal=Science |volume=287 |issue=5456 |pages=1265–9 |year=2000 |pmid=10678833 |doi=10.1126/science.287.5456.1265}}</ref> |
||
| − | Patients often begin with typical Parkinson's disease symptoms which persist for some years; these Parkinson-Plus diseases can only be diagnosed when other symptoms become apparent with the passage of time. These Parkinson-Plus diseases usually progress more quickly than typical ideopathic Parkinson disease. The usual anti-Parkinson's medications are typically either less effective or not effective at all in controlling symptoms; patients may be exquisitely sensitive to neuroleptic medications like haldol. Administration of such drugs is dangerous, even lethal, Additionally, the cholinesterase inhibiting medications have shown preliminary efficacy in treating the cognitive, psychiatric, and behavioral aspcects of the disease, so correct differential diagnosis is important. |
||
| − | == |
+ | == Diagnosis == |
| + | [[Image:PET scan Parkinson's Disease.jpg|thumb|200px|18F PET scan shows decreased dopamine activity in the [[basal ganglia]], a pattern which aids in diagnosing Parkinson's disease.]] |
||
| + | Typically, the diagnosis is based on medical history and neurological examination conducted by interviewing and observing the patient in person using the [[Unified Parkinson's Disease Rating Scale]]. A radiotracer for [[SPECT]] scanning machines called [[DaTSCAN]] and made by [[General Electric]] is specialized for diagnosing Parkinson's Disease, but it is only marketed in Europe. Due to this, the disease can be difficult to diagnose accurately, especially in its early stages. Due to symptom overlap with other diseases, only 75% of clinical diagnoses of PD are confirmed to be idiopathic PD at autopsy.<ref>{{cite journal | author = Gelb D, Oliver E, Gilman S | title = Diagnostic criteria for Parkinson disease | journal = Arch Neurol | volume = 56 | issue = 1 | pages = 33–9 | year = 1999 | pmid = 9923759 | doi = 10.1001/archneur.56.1.33}}</ref> Early signs and symptoms of PD may sometimes be dismissed as the effects of normal aging. The physician may need to observe the person for some time until it is apparent that the symptoms are consistently present. Usually doctors look for shuffling of feet and lack of swing in the arms. Doctors may sometimes request brain scans or laboratory tests in order to rule out other diseases. However, CT and MRI brain scans of people with PD usually appear normal. |
||
| + | [[Clinical practice guideline]]s introduced in the [[United Kingdom|UK]] in 2006 state that the diagnosis and follow-up of Parkinson's disease should be done by a specialist in the disease, usually a [[neurology|neurologist]] or [[geriatrician]] with an interest in movement disorders.<ref name=nice35>{{NICE|35|Parkinson's disease|June 2006}}</ref> |
||
| − | ===Distribution of Neural Degeneration in the Brain=== |
||
| − | The most striking gross pathologic abnormality in Parkinson disease (PD) is loss of pigmentation in an area of the [[midbrain]] called the [[substantia nigra]] pars compacta. This depigmentation corresponds to loss of [[neuromelanin]]-containing, [[dopamine]]-producing neurons. In the past, most of the symptoms of PD have been attributed to neuron loss in this region. |
||
| + | ==Treatment== |
||
| − | Pathologic abnormalities have also been noted in other regions of the brain. The [[Dutch]] neuropathologist Heiko Braak has described a progressive, upward involvement of brain structures, starting in the [[dorsal]] motor [[nucleus]] of the [[vagus nerve]] in the [[brainstem]], progressing over time to involve midbrain, [[limbic]], and finally [[cortex|neocortical]] neurons. Based on this work, Braak and his colleagues have proposed the following neuropathological staging system for PD: |
||
| + | Parkinson's disease is a chronic disorder that requires broad-based management including patient and family education, support group services, general wellness maintenance, [[physiotherapy]], exercise, and nutrition.<ref name=nice35/> At present, there is no cure for PD, but medications or surgery can provide relief from the symptoms. |
||
| + | ===Levodopa=== |
||
| − | Pre-clinical (no symptoms of PD). Neural degeneration confined to [[brainstem]]. |
||
| + | [[Image:Stalevo.jpg|thumb|250px|Stalevo for treatment of Parkinson's disease]] |
||
| − | *Stage 1: Dorsal motor nucleus of the vagus nerve |
||
| + | The most widely used form of treatment is [[L-dopa]] in various forms. L-dopa is transformed into dopamine in the dopaminergic neurons by L-aromatic amino acid decarboxylase (often known by its former name dopa-decarboxylase). However, only 1-5% of L-DOPA enters the dopaminergic neurons. The remaining L-DOPA is often metabolised to dopamine elsewhere, causing a wide variety of side effects. Due to feedback inhibition, L-dopa results in a reduction in the endogenous formation of L-dopa, and so eventually becomes counterproductive. |
||
| − | *Stage 2: [[Locus ceruleus]] and [[raphe nucleus]] |
||
| + | [[Carbidopa]] and [[benserazide]] are dopa decarboxylase inhibitors. They help to prevent the metabolism of L-dopa before it reaches the dopaminergic neurons and are generally given as combination preparations of [[carbidopa/levodopa]] (co-careldopa) (e.g. Sinemet, Parcopa) and [[benserazide|benserazide/levodopa]] (co-beneldopa) (e.g. Madopar). There are also controlled release versions of Sinemet and Madopar that spread out the effect of the L-dopa. Duodopa is a combination of levodopa and carbidopa, dispersed as a viscous gel. Using a patient-operated portable pump, the drug is continuously delivered via a tube directly into the upper small intestine, where it is rapidly absorbed. There is also Stalevo (Carbidopa, Levodopa and Entacapone). |
||
| − | Clinical (symptomatic). Progressive involvement of midbrain, limbic structures, and cortex. |
||
| − | *Stage 3: substantia nigra, [[amygdala]], basal forebrain (esp. nucleus basalis of Meynert), [[ventral tegmental area]], among others |
||
| − | *Stage 4: [[stria terminalis]], intralaminar [[thalamus|thalamic]] nuclei, [[insula|insular]] cortex, [[temporal]] [[mesocortex]], anterior [[cingulate gyrus]] |
||
| − | *Stage 5: neocortical higher-order [[sensory association areas]] and [[prefrontal fields]] |
||
| − | *Stage 6: first-order sensory association areas, [[premotor]] fields, primary neocortical fields |
||
| − | [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15338272&query_hl=1&itool=pubmed_DocSum] |
||
| + | ===COMT Inhibitors=== |
||
| − | Although there is still some degree of controversy surrounding this staging system, the broad areas of brain involved do seem to account for the myriad motor and non-motor symptoms found in PD. Braak and colleagues have embarked on the work of systematically correlating pathologic stage of PD with clinical manifestations. Recently, his team published work correlating the incidence and severity of dementia with pathologic stage of PD.[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15851731&query_hl=1&itool=pubmed_DocSum] |
||
| + | [[Tolcapone]] inhibits the [[COMT]] enzyme, thereby prolonging the effects of L-dopa, and so has been used to complement L-dopa. However, due to its possible side effects such as liver failure, it's limited in its availability. A similar drug, [[entacapone]] has not been shown to cause significant alterations of liver function and maintains adequate inhibition of COMT over time.<ref name=TNcilia>{{cite web | author=R. Cilia ''et al.''|year=2006 | title=Long-term Efficacy of Entacapone in Patients with Parkinson's Disease and Motor Fluctuations - A Six-Year Clinical Follow-Up Study |url=http://www.touchneurology.com/articles.cfm?article_id=5728&level=2}}</ref> |
||
| − | === |
+ | ===Dopamine agonists=== |
| + | The dopamine agonists [[bromocriptine]], [[pergolide]], [[pramipexole]], [[ropinirole]] , [[cabergoline]], [[apomorphine]], and [[lisuride]] are moderately effective. These have their own side effects including those listed above in addition to somnolence, hallucinations and/or insomnia. Several forms of dopamine agonism have been linked with a markedly increased risk of problem gambling. Dopamine agonists initially act by stimulating some of the dopamine receptors. However, they cause the dopamine receptors to become progressively less sensitive, thereby eventually increasing the symptoms. |
||
| − | Historically, the [[Lewy body]] has been the microscopic pathologic hallmark of Parkinson disease. Lewy bodies are found in the [[cytoplasm]] of neurons, and are composed of densely aggregated [[filament|filaments]]. These filaments contain [[ubiquitin]] and [[alpha-synuclein]]. |
||
| + | Dopamine agonists can be useful for patients experiencing on-off fluctuations and dyskinesias as a result of high doses of L-dopa. Apomorphine can be administered via subcutaneous injection using a small pump which is carried by the patient. A low dose is automatically administered throughout the day, reducing the fluctuations of motor symptoms by providing a steady dose of dopaminergic stimulation. After an initial "apomorphine challenge" in hospital to test its effectiveness and brief patient and [[primary caregiver]] (often a spouse or partner), the latter of whom takes over maintenance of the pump. The injection site must be changed daily and rotated around the body to avoid the formation of [[Nodule (medicine)|nodules]]. Apomorphine is also available in a more acute dose as an [[autoinjector]] pen for emergency doses such as after a fall or first thing in the morning. Nausea and vomiting are common, and may require [[domperidone]] (an antiemetic). |
||
| − | Patients with [[parkin]] mutations (PARK2, see below) do not have Lewy bodies. Such patients develop a syndrome that closely resembles the sporadic form of PD; however, they tend to develop symptoms at a much younger age. Whether the Lewy body itself causes neurodegeneration; or whether it is a protective response by damaged neurons is the focus of current research. |
||
| + | === MAO-B inhibitors === |
||
| − | ==Pathophysiology: A Complex Interaction Between Genetics and Environment== |
||
| + | [[Selegiline]] and [[rasagiline]] reduce the symptoms by inhibiting monoamine oxidase-B (MAO-B), which inhibits the breakdown of dopamine secreted by the dopaminergic neurons. Metabolites of selegiline include L-amphetamine and L-methamphetamine (not to be confused with the more notorious and potent dextrorotary isomers). This might result in side effects such as insomnia. Use of L-dopa in conjunction with selegiline has increased mortality rates that have not been effectively explained. Another side effect of the combination can be [[stomatitis]]. One report raised concern about increased mortality when MAO-B inhibitors were combined with L-dopa;<ref>{{cite journal | author = Thorogood M, Armstrong B, Nichols T, Hollowell J | title = Mortality in people taking selegiline: observational study | journal = BMJ | volume = 317 | issue = 7153 | pages = 252–4 | year = 1998 | pmid = 9677215}}</ref> however subsequent studies have not confirmed this finding.<ref>{{cite journal | author = Marras C, McDermott M, Rochon P, Tanner C, Naglie G, Rudolph A, Lang A | title = Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort | journal = Neurology | volume = 64 | issue = 1 | pages = 87–93 | year = 2005 | pmid = 15642909}}</ref> Unlike other non selective [[monoamine oxidase inhibitors]], tyramine-containing foods do not cause a hypertensive crisis. |
||
| − | The cause of neuron loss Parkinson's disease is not fully understood([[idiopathic]]). There are, however, many theories. |
||
| + | ===Surgery and deep brain stimulation=== |
||
| − | ===Genetic=== |
||
| + | [[Image:Parkinson surgery.jpg|thumb|200px|Illustration showing an electrode placed deep seated in the brain]] |
||
| − | Parkinson disease (PD) is thought to be caused by some combination of genetic and environmental factors. Up to one third of PD cases run in families.[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=PubMed&cmd=Retrieve&dopt=Citation&list_uids=99093357] The rest are apparently sporadic cases. Inheritance may be [[Mendelian]], i.e., [[autosomal recessive]], [[autosomal dominant]], or [[x-linked]]. Mitochondrial inheritance has been postulated but not proven. Most familial cases, however, follow no clear inheritance pattern. |
||
| + | Treating Parkinson's disease with surgery was once a common practice, but after the discovery of levodopa, surgery was restricted to only a few cases. Studies in the past few decades have led to great improvements in surgical techniques, and surgery is again being used in people with advanced PD for whom drug therapy is no longer sufficient. |
||
| + | [[Deep brain stimulation]] is presently the most used surgical means of treatment, but other surgical therapies that have shown promise include surgical lesion of the [[subthalamic nucleus]]<ref>{{cite journal |author=Guridi J, Obeso JA |title=The subthalamic nucleus, hemiballismus and Parkinson's disease: reappraisal of a neurosurgical dogma |journal=Brain |volume=124 |issue=Pt 1 |pages=5–19 |year=2001 |pmid=11133783 | url=http://brain.oxfordjournals.org/cgi/content/full/124/1/5 |doi=10.1093/brain/124.1.5}}</ref> and of the internal segment of the [[globus pallidus]], a procedure known as [[pallidotomy]].<ref>{{cite journal |author=Fukuda M, Kameyama S, Yoshino M, Tanaka R, Narabayashi H |title=Neuropsychological outcome following pallidotomy and thalamotomy for Parkinson's disease |journal=Stereotactic and functional neurosurgery |volume=74 |issue=1 |pages=11–20 |year=2000 |pmid=11124660 |doi=}}</ref> |
||
| − | An affected individual is three to four times more likely than an unaffected individual to have a close relative with PD. Having a first degree relative (parent or sibling) with PD doubles or triples an individual's risk of PD relative to the general population. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8710070&query_hl=57&itool=pubmed_docsum] |
||
| + | ===Neurorehabilitation=== |
||
| − | In recent years, a number of specific genetic mutations causing PD have been discovered. However, these account for a minority of PD cases. |
||
| + | There is partial evidence that speech or mobility problems can improve with rehabilitation although studies are still scarce and of low quality.<ref name="pmid11687029">{{cite journal |
||
| + | |author=Deane KH, Jones D, Playford ED, Ben-Shlomo Y, Clarke CE |
||
| + | |title=Physiotherapy for patients with Parkinson's Disease: a comparison of techniques |
||
| + | |journal=Cochrane Database Syst Rev |
||
| + | |volume= |
||
| + | |issue=3 |
||
| + | |pages=CD002817 |
||
| + | |year=2001 |
||
| + | |pmid=11687029 |
||
| + | |doi=10.1002/14651858.CD002817 |
||
| + | |url= |
||
| + | }}</ref><ref name="pmid11406045">{{cite journal |
||
| + | |author=Deane KH, Whurr R, Playford ED, Ben-Shlomo Y, Clarke CE |
||
| + | |title=A comparison of speech and language therapy techniques for dysarthria in Parkinson's disease |
||
| + | |journal=Cochrane Database Syst Rev |
||
| + | |volume= |
||
| + | |issue=2 |
||
| + | |pages=CD002814 |
||
| + | |year=2001 |
||
| + | |pmid=11406045 |
||
| + | |doi=10.1002/14651858.CD002814 |
||
| + | |url= |
||
| + | }}</ref><ref name="pmid18181210">{{cite journal |
||
| + | |author=Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL |
||
| + | |title=The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis |
||
| + | |journal=Mov. Disord. |
||
| + | |volume=23 |
||
| + | |issue=5 |
||
| + | |pages=631–40 |
||
| + | |year=2008 |
||
| + | |month=April |
||
| + | |pmid=18181210 |
||
| + | |doi=10.1002/mds.21922 |
||
| + | |url= |
||
| + | }}</ref><ref name="pmid12132992">{{cite journal |
||
| + | |author=Schulz GM |
||
| + | |title=The effects of speech therapy and pharmacological treatments on voice and speech in Parkinson s disease: a review of the literature |
||
| + | |journal=Curr. Med. Chem. |
||
| + | |volume=9 |
||
| + | |issue=14 |
||
| + | |pages=1359–66 |
||
| + | |year=2002 |
||
| + | |month=July |
||
| + | |pmid=12132992 |
||
| + | |doi= |
||
| + | |url=http://www.bentham-direct.org/pages/content.php?CMC/2002/00000009/00000014/0003C.SGM |
||
| + | }}</ref> Regular physical exercise and/or therapy can be beneficial to the patient for maintaining and improving mobility, flexibility, strength, gait speed, and quality of life;<ref name="pmid18181210"/> and speech therapy may improve voice and speech function.<ref name="pmid12132992"/> One of the most widely practiced treatment for the speech disorders associated with Parkinson's disease is the [[Lee Silverman Voice Treatment]] (LSVT). LSVT focuses on increasing vocal loudness.<ref name="pmid17117354">{{cite journal |
||
| + | |author=Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG |
||
| + | |title=The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders |
||
| + | |journal=Semin Speech Lang |
||
| + | |volume=27 |
||
| + | |issue=4 |
||
| + | |pages=283–99 |
||
| + | |year=2006 |
||
| + | |month=November |
||
| + | |pmid=17117354 |
||
| + | |doi=10.1055/s-2006-955118 |
||
| + | |url= |
||
| + | }}</ref> |
||
| + | ==Prognosis== |
||
| − | Genetic forms that have been identified include: |
||
| + | PD is not considered to be a fatal disease by itself, but it progresses with time. The average life expectancy of a PD patient is generally lower than for people who do not have the disease.<ref>{{cite web | title = Parkinson's Disease | publisher = Mayo Clinic: College of Medicine | url = http://cancercenter.mayo.edu/mayo/research/parkinsons/ | accessdate = 2006-11-04 }}</ref> In the late stages of the disease, PD may cause complications such as choking, pneumonia, and falls that can lead to death. |
||
| − | *''PARK1'' ([[OMIM]] #168601) [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=168601], caused by mutations in the ''SNCA'' gene, which codes for the [[protein]] [[alpha-synuclein]]. PARK1 causes [[autosomal dominant]] Parkinson disease. So-called PARK4 is probably caused by triplication of ''SNCA''.[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=14593171] |
||
| + | |||
| − | *''PARK2'' (OMIM *602544) [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=602544], caused by mutations in protein [[Parkin (ligase)|parkin]]. Parkin mutations may be one of the most common known genetic causes of early-onset Parkinson disease. In one study, of patients with onset of Parkinson disease prior to age 40 (10% of all PD patients), 18% had parkin mutations, with 5% [[homozygous]] mutations.[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15266615&query_hl=29&itool=pubmed_docsum]. Patients with an [[autosomal recessive]] family history of parkinsonism are much more likely to carry parkin mutations if age at onset is less than 20 (80% vs. 28% with onset over age 40).[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=12891670] |
||
| + | The progression of symptoms in PD may take 20 years or more. In some people, however, the disease progresses more quickly. There is no way to predict what course the disease will take for an individual person. With appropriate treatment, most people with PD can live productive lives for many years after diagnosis. |
||
| − | * ''PARK3'' (OMIM %602404) [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=602404], mapped to 2p, autosomal dominant, only described in a few kindreds. |
||
| − | *''PARK5'', caused by mutations in the ''UCHL1'' gene (OMIM +191342) [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=191342] which codes for the protein [[ubiquitin carboxy-terminal hydrolase L1]] |
||
| − | *''PARK6'' (OMIM #605909) [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=605909], caused by mutations in ''PINK1'' (OMIM *608309) which codes for the protein PTEN-induced putative kinase 1. |
||
| − | *''PARK7'' (OMIM #606324) [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=606324], caused by mutations in [[DJ-1]][http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=602533] |
||
| − | *''PARK8'' (OMIM #607060)[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=607060], caused by mutations in [[LRRK2]] which codes for the protein [[dardarin]]. ''In vitro'', mutant LRRK2 causes protein aggregation and cell death, possibly through an interaction with parkin. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16352719&query_hl=31&itool=pubmed_DocSum] LRRK2 mutations, of which the most common is G2019S, cause autosomal dominant Parkinson disease, with a [[penetrance]] of nearly 100% by age 80.[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=15726496] G2019S is the most common known genetic cause of Parkinson disease, found in 1-6% of U.S. and European PD patients.[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=16311269] It is especially common in Ashkenazi Jewish patients, with a prevalence of 29.7% in familial cases and 13.3% in sporadic.[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=16436782] |
||
| − | *''PARK12'' (OMIM %300557), maps to the X chromosome |
||
| + | In at least some studies, it has been observed that mortality was significantly increased, and longevity decreased among nursing home patients as compared to community dwelling patients.<ref>{{cite journal |author=Goetz CG, Stebbins GT |title=Mortality and hallucinations in nursing home patients with advanced Parkinson's disease |journal=Neurology |volume=45 |issue=4 |pages=669–71 |year=1995 |pmid=7723953 |doi=}}</ref> |
||
| − | [[mitochondria|Mitochondrial]] DNA mutations may also play a role in PD. Dysfunction in mitochondrial Complex I has been found in autopsy specimens and platelets from PD patients. Certain mitochondrial DNA haplogroups have been associated with increased susceptibility for disease. However, no kindred has been identified that demonstrates a clear pattern of mitochondrial inheritance. |
||
| + | One commonly used system for describing how the symptoms of PD progress is called the [[Hoehn and Yahr scale]]. Another commonly used scale is the [[Unified Parkinson's Disease Rating Scale]] (UPDRS). This much more complicated scale has multiple ratings that measure motor function, and also mental functioning, behavior, mood, and activities of daily living. Both the Hoehn and Yahr scale and the UPDRS are used to measure how individuals are faring and how much treatments are helping them. It should be noted that neither scale is specific to Parkinson's disease; that patients with other illnesses can score in the Parkinson's range. |
||
| − | ===Toxins=== |
||
| − | One theory holds that the disease may result in many or even most cases from the combination of a genetically determined vulnerability to environmental [[toxin]]s along with exposure to those toxins [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12428721&dopt=Citation]. This hypothesis is consistent with the fact that Parkinson's disease is not distributed homogenously throughout the population: rather, its incidence varies geographically. The toxins most strongly suspected at present are certain [[pesticide]]s and industrial metals. [[MPTP]] is used as a model for Parkinson's as it can rapidly induce parkinsonian symptoms in human beings and other animals, of any age. Other toxin-based models employ PCBs, [http://www.medicalnewstoday.com/medicalnews.php?newsid=19791] [[paraquat]] [http://www.jbc.org/cgi/content/full/277/3/1641] (a herbicide) in combination with maneb, a fungicide [http://www.jneurosci.org/cgi/content/full/20/24/9207] [[rotenone]] [http://www.nature.com/neuro/journal/v3/n12/abs/nn1200_1301.html;jsessionid=FF396CFE70AF6FB466818BCAC2196047] (an insecticide), and specific organochlorine pesticides including dieldrin [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11728820&query_hl=19&itool=pubmed_docsum] and lindane [http://journalsonline.tandf.co.uk/(4ihw1be5q55gc2551fxcaw55)/app/home/contribution.asp?referrer=parent&backto=issue,2,5;journal,124,196;linkingpublicationresults,1:100675,1]. Numerous studies have found an increase in Parkinson disease in persons who consume rural well water; researchers theorize that water consumption is a proxy measure of pesticide exposure. In agreement with this hypothesis are studies which have found a dose-dependent an increase in PD in persons exposed to agricultural chemicals. |
||
| + | ==Epidemiology== |
||
| − | Almost all of the PD-causing toxins act on the [[mitochondrial]] [[NADH dehydrogenase|complex I]] of the [[electron transfer chain]], and sporadic PD cases have been found to have a partial loss of activity of this enzyme complex. Studies in [[cybrids (medical)|cybrids]] have found that [[mitochondrial DNA]], rather than nuclear DNA, is responsible for the dysfunction. Most recently, [[microheteroplasmic]] mutations in one of the mitochondrial complex I genes, ND5, were found to be sufficient to diagnose sporadic PD correctly in 27 out of 28 cases. While additional studies are needed, mitochondrial microheteroplasmic mutations may be the cause of the majority of PD cases. |
||
| + | According to some sources, Parkinsons disease is slightly less prevalent in the [[African-American]] community. The average crude prevalence is estimated at being from 120-180 out of 100,000 among the [[Caucasian race|Caucasian]] (white) community.<ref>http://holisticonline.com/Remedies/Parkinson/pd_who-gets.htm</ref> The [[Parsi people|Parsi]] community in [[Mumbai]], [[India]] suffers from particularly high rates of Parkinson's disease.<ref>http://archneur.ama-assn.org/cgi/content/abstract/45/12/1321</ref><ref>http://holisticonline.com/Remedies/Parkinson/pd_who-gets.htm</ref> |
||
| − | However, the ubiquity of agricultural chemical exposures makes it difficult to gauge the true extent of the problem. In the current state of knowledge about the origins of the disease, it appears that family history of the disease and (especially) multiple episodes of head-trauma-induced unconsciousness increase individual risk more than does pesticide exposure, but research is continuing. |
||
| − | == |
+ | ==History== |
| + | Symptoms of Parkinson's disease have been known and treated since medieval times, most notably by [[Averroes]].<ref>{{cite journal |author=Manyam BV, Sánchez-Ramos JR |title=Traditional and complementary therapies in Parkinson's disease |journal=Advances in neurology |volume=80 |pages=565–74 |year=1999 |pmid=10410773}}</ref> However, it was not formally recognized and its symptoms were not documented until 1817 in ''An Essay on the Shaking Palsy''<ref>{{cite journal| author = Parkinson J | title = An essay on the shaking palsy. 1817 | journal = J Neuropsychiatry Clin Neurosci | volume = 14 | issue = 2 | pages = 223–36; discussion 222 | year = 2002 | pmid = 11983801| url=http://neuro.psychiatryonline.org/cgi/content/full/14/2/223|format=Reproduced | doi = 10.1176/appi.neuropsych.14.2.223}}</ref> |
||
| − | Past episodes of head trauma are reported more frequently by sufferers than by others in the population [http://www.neurology.org/cgi/content/abstract/60/10/1610][http://archneur.ama-assn.org/cgi/content/abstract/48/9/903][http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14637093&query_hl=3&itool=pubmed_DocSum]. A methodologically strong recent study [Bower 2003] found that those who have experienced a head injury are four times more likely to develop Parkinson’s disease than those who have never suffered a head injury. The risk of developing Parkinson’s increases eightfold for patients who have had head trauma requiring hospitalization, and it increases 11-fold for patients who have experienced severe head injury[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=14637093&query_hl=3&itool=pubmed_DocSum]. While emotional or psychological [[Psychological trauma|trauma]] can precipitate the initial symptoms or aggravate existing symptoms, this is probably not the actual cause of the disorder. However, psychological trauma during periods of developmental susceptibility cannot be definitely excluded as triggers. |
||
| + | by the British physician [[James Parkinson]]. Parkinson's disease was then known as ''paralysis agitans'', the term "Parkinson's disease" being coined later by [[Jean-Martin Charcot]]. The underlying [[biochemical]] changes in the [[brain]] were identified in the 1950s due largely to the work of Swedish scientist [[Arvid Carlsson]], who later went on to win a [[Nobel Prize]]. [[L-dopa]] entered clinical practice in 1967,<ref>{{cite journal |author=Hornykiewicz O |title=L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent |journal=Amino Acids |volume=23 |issue=1-3 |pages=65–70 |year=2002 |pmid=12373520 |doi=10.1007/s00726-001-0111-9}}</ref> and the first large study reporting improvements in patients with Parkinson's disease resulting from treatment with L-dopa was published in 1968.<ref>{{cite journal| author = Cotzias G | title = L-Dopa for Parkinsonism | journal = N Engl J Med | volume = 278 | issue = 11 | pages = 630 | year = 1968 | pmid= 5637779}}</ref> |
||
| + | ==Research directions== |
||
| − | Other Associations |
||
| + | ===Gene therapy=== |
||
| + | Currently under investigation is gene therapy. This involves using a non-infectious virus to shuttle a gene into a part of the brain called the subthalamic nucleus (STN). The gene used leads to the production of an enzyme called glutamic acid decarboxylase ([[Glutamate decarboxylase|GAD]]), which catalyses the production of a [[neurotransmitter]] called [[GABA]].<!-- |
||
| + | --><ref name="pmid17586305">{{cite journal |author=Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ |title=Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial |journal=Lancet |volume=369 |issue=9579 |pages=2097–105 |year=2007 |pmid=17586305 |doi=10.1016/S0140-6736(07)60982-9}}</ref><!-- |
||
| − | * [[Prior history of an affective disorder]] [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11722320&query_hl=4&itool=pubmed_docsum] |
||
| + | --> GABA acts as a direct inhibitor on the overactive cells in the STN. |
||
| + | [[GDNF]] infusion involves the infusion of GDNF (glial-derived neurotrophic factor) into the basal ganglia using surgically implanted catheters. Via a series of biochemical reactions, GDNF stimulates the formation of L-dopa. GDNF therapy is still in development. |
||
| − | ===Loss of dopamine-secreting cells=== |
||
| − | The symptoms of Parkinson's disease result from the loss of [[dopamine]]-secreting (dopaminergic) cells and subsequent loss of [[melanin]], secreted by the same cells, in the [[substantia nigra|pars compacta]] region of the [[substantia nigra]] (literally "black substance"). These neurons project to the [[striatum]] and their loss leads to alterations in the activity of the neural circuits within the basal ganglia that regulate movement, in essence an inhibition of the [[direct pathway]] and excitation of the [[indirect pathway]]. |
||
| + | Implantation of stem cells genetically engineered to produce dopamine or stem cells that transform into dopamine-producing cells has already started being used. These could not constitute cures because they do not address the considerable loss of activity of the dopaminergic neurons. Initial results have been unsatisfactory, with patients still retaining their drugs and symptoms. |
||
| − | The direct pathway facilitates movement and the indirect pathway inhibits movement, thus the loss of these cells leads to a hypokinetic movement disorder. The lack of [[dopamine]] results in increased inhibition of the ventral lateral nucleus of the thalamus, which sends excitatory projections to the motor cortex, thus leading to [[hypokinesia]]. |
||
| + | === Neuroprotective treatments === |
||
| − | There are four major dopamine pathways in the brain; the nigrostiatal pathway, referred to above, mediates movement and is the most conspicuously affected in early Parkinson's disease. The other pathways are the mesocortical, the mesolimbic, and the tuberoinfundibular. These pathways are associated with, respectively: volition and emotional responsiveness; desire, initiative, and reward; and sensory processes and maternal behavior. Disruption of dopamine along the non-striatal pathways is the likely explantion for much of the neuropsychiatric pathology associated with Parkinson's disease. |
||
| + | [[Neuroprotective]] treatments are at the forefront of PD research, but are still under clinical scrutiny.<ref> {{cite journal| author=Bonuccelli U, |
||
| + | Del Dotto P| title= New pharmacologic horizons in the treatment of Parkinson disease | journal=Neurology | year=2006 | volume=67 | issue=2 | pages= 30–38}}</ref> These agents could protect neurons from cell death induced by disease presence resulting in a slower progression of disease. Agents currently under investigation as neuroprotective agents include anti-apoptotic drugs (CEP 1347 and CTCT346), lazaroids, bioenergetics, antiglutamatergic agents and dopamine receptors.<ref>{{cite journal |author=Djaldetti R, Melamed E |title=New drugs in the future treatment of Parkinson's disease |journal=J. Neurol. |volume=249 Suppl 2 |issue= |pages=II30–5 |year=2002 |pmid=12375061 |doi=10.1007/s00415-002-1206-2}}</ref> Clinically evaluated neuroprotective agents are the monoamine oxidase inhibitors selegiline<ref name=PSG_1993>{{cite journal |author= |title=Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group |journal=N. Engl. J. Med. |volume=328 |issue=3 |pages=176–83 |year=1993 |pmid=8417384 |doi=}}</ref> and rasagiline, dopamine agonists, and the complex I mitochondrial fortifier [[coenzyme Q10]]. |
||
| + | === Neural transplantation === |
||
| − | Brain cells producing other brain chemicals such as [[GABA]], [[norepinephrine]], [[serotonin]] and [[acetylcholine]] exhibit damage in Parkinson's disease, accounting for some of the wide array of symptoms.It is not known whether this damage is a primary disease process, or secondary to loss of normal dopaminergic stimulation. |
||
| + | The first prospective randomised double-blind sham-placebo controlled trial of dopamine-producing cell transplants failed to show an improvement in quality of life although some significant clinical improvements were seen in patients below the age of 60.<ref> {{cite journal |author=Freed CR, Greene PE, Breeze RE, ''et al'' |title=Transplantation of embryonic dopamine neurons for severe Parkinson's disease |journal=N. Engl. J. Med. |volume=344 |issue=10 |pages=710–9 |year=2001 |pmid=11236774 |doi=}}</ref> A significant problem was the excess release of dopamine by the transplanted tissue, leading to [[dystonia]]s.<ref>{{cite journal |author=Redmond DE |title=Cellular replacement therapy for Parkinson's disease--where we are today? |journal=The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry |volume=8 |issue=5 |pages=457–88 |year=2002 |pmid=12374430 |doi=}}</ref> Research in African [[green monkey]]s suggests that the use of [[stem cell]]s might in future provide a similar benefit without inducing dystonias.<ref>{{cite journal |author=Redmond E et al |title=Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells |journal=Proceedings of the National Academy of Sciences |volume=104 |issue=29 |year=2007 |doi=10.1073/pnas.0704091104 |pages=12175 |pmid=17586681}}</ref> |
||
| + | === Alternative Treatments === |
||
| − | The mechanism by which the brain cells in Parkinson's are lost appears to center on an abnormal accumulation of the protein [[alpha-synuclein]] bound to ubiquitin in the damaged cells.[[alpha-synuclein]]-ubiquitin complex cannot be directed to the proteosome. This protein accumulation forms proteinaceous cytoplasmic inclusions called [[Lewy bodies]]. Excessive accumulations of iron, which are toxic to nerve cells, are also typically observed in conjunction with the protein inclusions. |
||
| + | Nutrients have been used in clinical studies and are used by people with PD in order to partially treat PD or slow down its deterioration. The L-dopa precursor [[L-tyrosine]] was shown to relieve an average of 70% of symptoms.<ref>{{cite journal | author=Lemoine P, Robelin N, Sebert P, Mouret J | title=La L-tyrosine : traitement au long cours de la maladie de Parkinson [L-tyrosine : A long term treatment of Parkinson's Disease] | journal=Comptes rendus academie des sciences | year=1986 | volume=309 | issue= | pages=43–47 | language=French }}</ref> Ferrous iron, the essential cofactor for L-dopa biosynthesis was shown to relieve between 10% and 60% of symptoms in 110 out of 110 patients.<ref>{{cite journal |author=Birkmayer W, Birkmayer JG |title=Iron, a new aid in the treatment of Parkinson patients |journal=J. Neural Transm. |volume=67 |issue=3-4 |pages=287–92 |year=1986 |pmid=3806082 | url=http://www.springerlink.com/link.asp?id=tp15r2g8u6327731 |doi=10.1007/BF01243354}}</ref> |
||
| + | <ref>{{cite book | editor= Editors Przuntek H , Riederer P | title=Early diagnosis and preventive therapy in Parkinson's disease | date=1989 | publisher= Springer | isbn = 0-387-82080-9 | pages=p. 323}}</ref> More limited efficacy has been obtained with the use of THFA, NADH, and pyridoxine—coenzymes and coenzyme precursors involved in dopamine biosynthesis.<ref>{{cite web | url = http://home.uchicago.edu/~syin/Kang.doc | title = Dopamine biosynthesis | accessdate = 2006-11-04 | format = Word doc | publisher = University of Chicago Personal Web Pages}}</ref> Vitamin C and vitamin E in large doses are commonly used by patients in order to theoretically lessen the cell damage that occurs in PD. This is because the enzymes superoxide dismutase and catalase require these vitamins in order to nullify the superoxide anion, a toxin commonly produced in damaged cells. However, in the randomized controlled trial, DATATOP of patients with early PD, no beneficial effect for vitamin E compared to placebo was seen.<ref name=PSG_1993 /> Coenzyme Q10 has more recently been used for similar reasons. MitoQ is a newly developed synthetic substance that is similar in structure and function to coenzyme Q10. |
||
| + | Studies looking at [[qigong]] in PD have not reached consensus on its efficacy.<ref>{{cite journal | author = Schmitz-Hubsch T | title = Qigong exercise for the symptoms of Parkinson's disease: a randomized, controlled pilot study | journal = Mov Disord | volume = 21 | issue = 4 | pages = 543–548 | year = 2006 | pmid = 16229022 | doi = 10.1002/mds.20705}}</ref><ref>{{cite journal |author=Burini D, Farabollini B, Iacucci S, ''et al'' |title=A randomised controlled cross-over trial of aerobic training versus qigong in advanced Parkinson's disease |journal=Europa medicophysica |volume=42 |issue=3 |pages=231–8 |year=2006 |pmid=17039221 |doi=}}</ref> |
||
| − | The precise mechanism whereby aggregates of alpha-synuclein damage the cells is not known. The aggregates may be merely a normal reaction by the cells as part of their effort to correct a different, as-yet unknown, insult. It does appear that alpha-synuclein aggregation is enhanced by the presence of dopamine and the byproducts of dopamine production. Based on this mechanistic hypothesis, a [[Transgenic animal|transgenic mouse model]] of Parkinson's has been generated by introduction of human wild-type α-synuclein into the mouse genome under control of the [[Platelet-derived growth factor|platelet-derived-growth factor]]-β promoter.{{ref|PDmousemodel}} |
||
| + | ''[[Mucuna pruriens]]'', is a natural source of therapeutic quantities of L-dopa, and has been under some investigation.<ref>{{cite journal |author=Katzenschlager R, Evans A, Manson A, ''et al'' |title=Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study |journal=J. Neurol. Neurosurg. Psychiatr. |volume=75 |issue=12 |pages=1672–7 |year=2004 |pmid=15548480 |doi=10.1136/jnnp.2003.028761}}</ref> |
||
| − | ==Treatment== |
||
| − | The treatment of Parkinson's disease mainly relies on replacing [[dopamine]] with [[levodopa]] (L-DOPA) or mimicking its action with dopamine [[agonist]]s such as [[pramipexole]], [[ropinirole]], [[pergolide]] or [[bromocriptine]]. Discovered as a Parkinson's treatment by [[Arvid Carlsson]], levodopa is a dopamine precursor that is transfomed into dopamine by dopa-decarboxylase, present in the basal ganglia in the [[brain]] as well as other tissues, e.g., the retina. Levodopa is almost always supplemented with [[carbidopa]] or [[benserazide]], dopa-decarboxylase inhibitors which prevent levodopa from being prematurely converted into dopamine in the adrenal glands or other peripheral tissues. This leaves more levodopa to reach the brain and allows a reduced dosage to produce the same effect on the central nervous system, thus reducing the peripheral side effects, which included protracted nausea, before the formulation l-dopa with carbidopa. |
||
| + | ==See also== |
||
| − | The most frequent side effects of these dopaminergic drugs are nausea, sleepiness, dizziness, involuntary writhing movements and visual hallucinations. Despite these side effects, treatment of a Parkinson's patient with these two drugs can result a striking "return to life" in the eyes of the patient's family and doctors, to the point that the patient's illness is imperceptibe to most observers. |
||
| + | *[[Amantadine]] |
||
| + | *[[Antitremor drugs]] |
||
| + | *[[Parkinsonism]] |
||
| + | *[[Psychosis in parkinsons disease]] |
||
| + | *[[Tremor]] |
||
| + | ==References== |
||
| − | However, progression is inexorable, and the drugs are not effective forever. A point is reached where the drugs only work for periods of a few hours ("on" periods) which are sandwiched between longer interval during which the drugs are partially or completely ineffective ("off periods"). |
||
| + | <!--See [[Psychological wiki:Footnotes]] for an explanation of how to generate footnotes or references using the <references/)> tags--> |
||
| + | <div class="references-small" style="-moz-column-count:2; column-count:2;"> |
||
| + | <references/></div> |
||
| + | ==External links== |
||
| − | With protracted use of dopamine replacement, the intervals of partial effectiveness are characterized by writhing, choreiform movements --dyskinesia-- that are involuntary, can be very disruptive of normal movement and communication, and can physically exhaust patients. "On", "off", and "dyskinesia" are motor fluctuations: Almost all patients who use dopamine replacement therapy develop motor fluctuations within ten years, younger patients more quickly than older. Despite their somewhat bizarre appearance, a majority of patients prefer a state of dyskinetic "on" to immobilized "off", possibly because the off state is so subjectively paralyzing, and often accompanied by panic. |
||
| + | {{commonscat|Parkinson's disease}} |
||
| + | {{wikibooks|Speech-Language Pathology}} |
||
| + | * {{dmoz|Health/Conditions_and_Diseases/Neurological_Disorders/Parkinson's_Disease/|Parkinson's Disease}} |
||
| + | * [http://www.ninds.nih.gov/disorders/parkinsons_disease/parkinsons_disease.htm Parkinson's Disease: Hope Through Research (National Institute of Neurological Disorders and Stroke)] |
||
| + | * [http://www.wpda.org/ World Parkinson Disease Association] |
||
| − | [[Therapy]] for Parkinson disease typically requires an evolving regimen of multiple medications, each calibrated to individual physiology and symptoms. Medicating to control the side effects of other medications contributes to polypharmacy: [[Amantadine hydrochloride]], [[anticholinergic]]s and [[COMT inhibitor]]s [[tolcapone]] or [[entacapone]] are sometimes prescribed to reduce the motor fluctuations that are a consequence of dopaminergic therapy. Tolcapone should be used with extreme caution because of the possibility of liver failure; Entacapone has not been shown to cause significant alterations of liver function. |
||
| − | Foods rich in proteins can reduce the uptake of levodopa, because some [[amino acid]]s compete with levodopa for cellular receptor sites. This can usually be dealt with by offsetting medication and meal times: consuming the majority of required proteins towards the evening allows patients to use dopamine medication more effectively during the morning and mid-day when mobility is more critical. |
||
| + | {{Mental and behavioural disorders}} |
||
| − | While these therapies are a good attempt at treating the symptoms, they are not a cure--they do not attack the underlying cause of the disease which is a loss of dopamine producing neurons. Only a therapy that addresses the underlying causes of dopamine cell pathology and permits their replacemt is likely to constute a cure. |
||
| + | {{Diseases of the nervous system}} |
||
| + | [[Category:Aging-associated diseases]] |
||
| − | Regular physical exercise and/or therapy are beneficial to the patient and essential for maintaining and improving mobility, flexibility, balance and a range of motion, and for a better resistance against many of the secondary symptoms and side effects. There is increasing evidence that exercise is both neuroprotective against the development of Parkinson's disease, and also ameliorative of both severity of symptoms, and also possibly of progression. "Alternative" exercise modalities such as yoga, tai chi, and dance may also hold promise as rehabilitation therapies, due to their integration of movement, thought, feeling, and sensory experience. Exercise has also been shown to effectively improve mild-moderate/ depression, |
||
| + | [[Category:Brain disorders]] |
||
| − | |||
| − | Surgical interventions are an active area of current research, and [[deep brain stimulation]] is presently the most popular and effective such treatment. In the future, implantation of cells genetically engineered to produce dopamine or stem cells that transform into dopamine-producing cells may become available. |
||
| − | |||
| − | Even these, however, will not constitute cures because they do not address the widespread loss of several different types of cells in the brain and even for the dopamine-producing cells, do not re-establish all of the original connections with neighboring brain cells. A true cure will have to detect the earliest signs of the disorder before they cause important symptoms and will intervene in the process that damages the brain cells in the first place. |
||
| − | |||
| − | Dopamine deficiency is central, but deficits of serotonin, norepinephrine, and acetylcholine are also typical. The depression and anxiety states that predominate when serotonin and norepinephrine are deficient are often treated with selective serotonine reuptake inhibitors (SSRIs) like Paxil, Zoloft, or Celexa; there is emerging evidence that the SSNRI (selective serotonin and norepinephrine reuptake inhibitor) Effexor may be particularly effective in Parkinson's disease because it augments two deficient neurotransmitters. Amphetimine-like drugs (Ritalin, Concerta) are being prescribed with increasing frequency to treat the Attention Deficit Disorder (ADD)-like attention problems that are almost universal in Parkinson's disease. Finally, there is emerging evidence to suggest that drugs that inhibit the reuptake of acetylcholine, developed as treatments for Alzheimer's dementia, may also improve memory and executive function in Parkinson's disease. |
||
| − | |||
| − | The patient and physicians are confronted with the behavioral and cognitive consequences of disruptions in at least five neurotransmitters; in addition to the four above, GABA is also disrupted. The inevitable cost, risk, and sheer unpleasantness of such complex medication regimens drives both doctors and patients to advocate for better and more comprehensive therapies. |
||
| − | |||
| − | The best evidence is that analytic and synthetic reasoning are relatively spared, even in advancing Parkinson's disease. However, the evidence that executive function impairment begins early and is progressive is growing rapidly. Coupled with the observation that more than 70 percent of Parkinson disease patients meet the criteria for at least one psychiatric diagnosis (most commonly anxiety or depression, with apathy also significant), the picture that emerges is one of considerable neuropsychological disability in individuals with preserved reasoning and awareness. |
||
| − | |||
| − | Because reasoning and awareness are operative, most patients can and should participate in their own care. This is correct from a legal and moral perspective of respect for the dignity and autonomy of individual patients, but it is also good medical practice. The formation of a "therapeutic alliance" between the patient and the physician ensures the optimal exchange of information, and amplifies the effectiveness of medical interventions. |
||
| − | |||
| − | The liberty to exercise preferences, even in regard to seemingly trivial details, has been shown to preserve the intellectual and emotional integrity of very physically compromised individuals. Patients have both a legal and a moral right to participate in their own care to the fullest extent possible. |
||
| − | |||
| − | The cumulative prevalence of dementia (substantially disabling defects in memory and reasoning)in Parkinson disease is still being debated, but the estimates range from 40 to 80 percent; more careful analysis seems to support the higher estimates. . With this fact in mind, patients, families, caregivers and medical personnel should work together to outline clear and pragmatically possible ways to preserve the dignity and choices of patients even when they cannot speak clearly for themselves. |
||
| − | |||
| − | |||
| − | ===Secondary parkinsonism=== |
||
| − | '''Secondary parkinsonism''' (or briefly '''parkinsonism''') is a term used for a symptom constellation that is similar to that of Parkinson's disease but is caused by other disorders or medications. Major reasons for secondary parkinsonism are [[stroke]], [[encephalitis]], [[narcotic]]s, [[toxins]] such as [[manganese]] or [[carbon monoxide poisoning]], traumatic brain injury, and [[normal pressure hydrocephalus]]. |
||
| − | |||
| − | There are other [[idiopathic]] (of unknown cause) conditions as Parkinson's disease that may cause parkinsonism. In these conditions the problem is not the deficient production of dopamine but the inefficient binding of dopamine to its receptors located on [[globus pallidus]]. |
||
| − | |||
| − | ==Parkinson's and death== |
||
| − | Parkinson's does not by itself cause death, but since the disease may affect the [[respiratory system]], sufferers may eventually contract [[pneumonia]] and die. Swallowing difficulties may lead to [[aspiration]] of food, causing [[aspiration pneumonia]] (a specific form of pneumonia caused by gastric acid, food and digestive tract bacteria) . Immobility may increase susceptibility to [[infection]]. Onset of dementia doubles the odds of death; depression more than doubles the odds ratio. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15242420&query_hl=11&itool=pubmed_docsum] However, people may live for 20 to 30 years with the condition. |
||
| − | |||
| − | ==Notable Parkinson's sufferers== |
||
| − | One famous sufferer of young-onset Parkinson's is [[Michael J. Fox]], who has written a book about his experience of the disease. The film ''[[Awakenings]]'' (starring [[Robin Williams]] and [[Robert De Niro]] and based on genuine cases reported by [[Oliver Sacks]]) deals sensitively and largely accurately with a similar disease, [[postencephalitic parkinsonism]]; the state of the art in treatment remains roughly the same as it was at the time of the events depicted, the [[1960s]], although patients with postencephalitic parkinsonism lose benefit from their medication far faster than do patients with Parkinson's disease. Other famous sufferers include [[Pope John Paul II]], former US Attorney General [[Janet Reno]], and dictator [[Adolf Hitler]] (who may have developed Parkinsonism as a consequence of poisonous gas exposures in the trenches of WWI). |
||
| − | |||
| − | ==See also== |
||
| − | * [[List of famous Parkinson's disease patients]] |
||
| − | * [[Contursi]] |
||
| − | |||
| − | ==External links== |
||
| − | *[http://www.liebermanparkinsonclinic.com/ Dr. Abe Lieberman's Ask the Doctor Parkinson Forum] |
||
| − | *[http://www.pdf.org/ Parkinson's Disease Foundation] |
||
| − | *[http://www.parkinson.org/ National Parkinson Foundation, Inc.] |
||
| − | *[http://www.apdaparkinson.org/user/index.asp American Parkinson's Disease Association] |
||
| − | *[http://www.michaeljfox.org/ Michael J. Fox Foundation for Parkinson's Research] |
||
| − | *[http://www.parkinsons.org.uk/ Parkinson's Disease Society (UK)] |
||
| − | *[http://www.parkinsonsnsw.org.au/ Parkinson's NSW (In AUS)] |
||
| − | *[http://www.MyParkinsonsInfo.com/ MyParkinsonsInfo.com] |
||
| − | *[http://www.pdring.com/ The PD Webring] |
||
| − | *[http://www.hollyrod.org/ The HollyRod Foundation] |
||
| − | *[http://www.wemove.org/ WEMOVE: Worldwide education and awareness for movement disorders] |
||
| − | *[http://www.nwpf.org/ Northwest Parkinson's Foundation] |
||
| − | *[http://www.geocities.com/murraycharters Parkinson's Resources on the WWWeb] |
||
| − | *[http://health.allrefer.com/health/parkinsons-disease-info.html Parkinson's Disease Pictures] |
||
| − | *[http://www.rxfiles.ca/acrobat/Parkinsons-Complete-Header.pdf Parkinson's Drug Comparison Chart (PDF)] |
||
| − | *[http://www.jpgmonline.com/article.asp?issn=0022-3859;year=2003;volume=49;issue=3;spage=236;epage=245;aulast=Kedar Can We Prevent Parkinson’s and Alzheimer’s Disease?] Article from Journal of Postgraduate Medicine |
||
| − | *[http://www.ispgr.org/ International Society for Posture and Gait Research (ISPGR)] |
||
| − | |||
| − | ==References== |
||
| − | # {{note|PDmousemodel}} {{cite journal |
||
| − | | author=Eliezer Masliah, Edward Rockenstein, Isaac Veinbergs, Margaret Mallory, Makoto Hashimoto, Ayako Takeda, Yutaka Sagara, Abbyann Sisk, Lennart Mucke |
||
| − | | title= Dopaminergic Loss and Inclusion Body Formation in alpha-Synuclein Mice: Implications for Neurodegenerative Disorders |
||
| − | | journal=Science |
||
| − | | volume=287 | issue=5456 | year=2000 | pages= 1265–1269 |
||
| − | | id=PMID 10678833 |
||
| − | }} |
||
| − | |||
| − | [[Category:Diseases]] |
||
| − | [[Category:Eponymous diseases]] |
||
[[Category:Geriatrics]] |
[[Category:Geriatrics]] |
||
| − | [[Category: |
+ | [[Category:Neuromuscular disorders]] |
| + | [[Category:Paralysis]] |
||
| + | [[Category:Parkinson's disease|*]] |
||
| + | <!-- |
||
| − | [[ar:مرض باركنسن]] |
||
| + | [[ar:مرض باركنسون]] |
||
| + | [[zh-min-nan:Parkinson ê pēⁿ]] |
||
| + | [[bs:Parkinsonova bolest]] |
||
[[ca:Malaltia de Parkinson]] |
[[ca:Malaltia de Parkinson]] |
||
| − | [[cs:Parkinsonova |
+ | [[cs:Parkinsonova nemoc]] |
[[da:Parkinsons sygdom]] |
[[da:Parkinsons sygdom]] |
||
[[de:Parkinson-Krankheit]] |
[[de:Parkinson-Krankheit]] |
||
| + | [[et:Parkinsoni tõbi]] |
||
[[el:Νόσος του Πάρκινσον]] |
[[el:Νόσος του Πάρκινσον]] |
||
[[es:Enfermedad de Parkinson]] |
[[es:Enfermedad de Parkinson]] |
||
[[eo:Parkinsono]] |
[[eo:Parkinsono]] |
||
| + | [[fa:پارکینسون]] |
||
[[fr:Maladie de Parkinson]] |
[[fr:Maladie de Parkinson]] |
||
| − | [[ |
+ | [[gl:Párkinson]] |
| + | [[ko:파킨슨병]] |
||
[[hr:Parkinsonova bolest]] |
[[hr:Parkinsonova bolest]] |
||
[[id:Penyakit Parkinson]] |
[[id:Penyakit Parkinson]] |
||
[[it:Morbo di Parkinson]] |
[[it:Morbo di Parkinson]] |
||
[[he:מחלת פרקינסון]] |
[[he:מחלת פרקינסון]] |
||
| + | [[ku:Nexweşiya parkinsonê]] |
||
| + | [[lb:Parkinson-Krankheet]] |
||
| + | [[ml:പാര്ക്കിന്സണ്സ് രോഗം]] |
||
[[ms:Penyakit Parkinson]] |
[[ms:Penyakit Parkinson]] |
||
[[nl:Ziekte van Parkinson]] |
[[nl:Ziekte van Parkinson]] |
||
| − | [[ja:パーキンソン |
+ | [[ja:パーキンソン病]] |
[[no:Parkinsons sykdom]] |
[[no:Parkinsons sykdom]] |
||
[[pl:Choroba Parkinsona]] |
[[pl:Choroba Parkinsona]] |
||
| − | [[pt: |
+ | [[pt:Doença de Parkinson]] |
[[ru:Болезнь Паркинсона]] |
[[ru:Болезнь Паркинсона]] |
||
| + | [[simple:Parkinson's disease]] |
||
| + | [[sr:Паркинсонова болест]] |
||
[[fi:Parkinsonin tauti]] |
[[fi:Parkinsonin tauti]] |
||
[[sv:Parkinsons sjukdom]] |
[[sv:Parkinsons sjukdom]] |
||
[[th:โรคพาร์กินสัน]] |
[[th:โรคพาร์กินสัน]] |
||
| + | [[uk:Хвороба Паркінсона]] |
||
[[zh:帕金森氏症]] |
[[zh:帕金森氏症]] |
||
| + | --> |
||
| − | |||
{{enWP|Parkinson's disease}} |
{{enWP|Parkinson's disease}} |
||
Revision as of 18:01, 17 May 2010
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Clinical: Approaches · Group therapy · Techniques · Types of problem · Areas of specialism · Taxonomies · Therapeutic issues · Modes of delivery · Model translation project · Personal experiences ·
Sir William Richard Gowers Parkinson Disease sketch 1886.jpg|
| |||||||||||||||
Parkinson's disease or PD (also known as Parkinson's syndrome or paralysis agitans ) is a neurodegenerative disorder of the central nervous system that often impairs the sufferer's motor skills, speech, and other functions.[1]
Parkinson's disease belongs to a group of conditions called movement disorders. It is characterized by muscle rigidity, tremor, a slowing of physical movement (bradykinesia) and, in extreme cases, a loss of physical movement (akinesia). The primary symptoms are the results of decreased stimulation of the motor cortex by the basal ganglia, normally caused by the insufficient formation and action of dopamine, which is produced in the dopaminergic neurons of the brain. Secondary symptoms may include high level cognitive dysfunction and subtle language problems. PD is both chronic and progressive.
PD is the most common cause of chronic progressive parkinsonism, a term which refers to the syndrome of tremor, rigidity, bradykinesia and postural instability. PD is also called "primary parkinsonism" or "idiopathic PD" (classically meaning having no known cause although this term is not strictly true in light of the plethora of newly discovered genetic mutations). While many forms of parkinsonism are "idiopathic", "secondary" cases may result from toxicity most notably of drugs, head trauma, or other medical disorders. The disease is named after English physician James Parkinson, who made a detailed description of the disease in his essay: "An Essay on the Shaking Palsy" (1817).
Classification
"Parkinson's disease" is the synonym of "primary parkinsonism", i.e. isolated parkinsonism due to a neurodegenerative process without any secondary systemic cause. In some cases, it would be inaccurate to say that the cause is "unknown", because a small proportion is caused by genetic mutations. It is possible for a patient to be initially diagnosed with Parkinson's disease but then to develop additional features, requiring revision of the diagnosis.[2]
There are other disorders that are called Parkinson-plus diseases. These include: multiple system atrophy (MSA), progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). Some include dementia with Lewy bodies (DLB) — while idiopathic Parkinson's disease patients also have Lewy bodies in their brain tissue, the distribution is denser and more widespread in DLB. Even so, the relationship between Parkinson disease, Parkinson disease with dementia (PDD), and dementia with Lewy bodies (DLB) might be most accurately conceptualized as a spectrum, with a discrete area of overlap between each of the three disorders. The natural history and role of Lewy bodies is little understood.
These Parkinson-plus diseases may progress more quickly than typical idiopathic Parkinson disease. If cognitive dysfunction occurs before or very early in the course of the movement disorder then DLBD may be suspected. Early postural instability with minimal tremor especially in the context of ophthalmoparesis should suggest PSP. Early autonomic dysfunction including erectile dysfunction and syncope may suggest MSA. The presence of extreme asymmetry with patchy cortical cognitive defects such as dysphasia and apraxias especially with "alien limb" phenomena should suggest CBD.
The usual anti-Parkinson's medications are typically either less effective or not effective at all in controlling symptoms; patients may be exquisitely sensitive to neuroleptic medications like haloperidol. Additionally, the cholinesterase inhibiting medications have shown preliminary efficacy in treating the cognitive, psychiatric, and behavioral aspects of the disease, so correct differential diagnosis is important.
Essential tremor may be mistaken for Parkinson's disease but lacks all other features besides tremor, and has particular characteristics distinguishing it from Parkinson's, such as improvement with beta blockers and alcoholic beverages.[1]
Wilson's disease (hereditary copper accumulation) may present with parkinsonian features; young patients presenting with parkinsonism or any other movement disorder are frequently screened for this rare condition, because it may respond to medical treatment. Typical tests are liver function, slit lamp examination for Kayser-Fleischer rings, and serum ceruloplasmin levels.
Signs and symptoms
Parkinson disease affects movement (motor symptoms). Other typical symptoms include disorders of mood, behaviour, thinking, and sensation (non-motor symptoms). Patients' individual symptoms may be quite dissimilar and progression of the disease is also distinctly individual.
Motor symptoms
The cardinal symptoms are (mnemonic "TRAP"):[1]
- Tremor: normally 4-6 Hz tremor, maximal when the limb is at rest, and decreased with voluntary movement. It is typically unilateral at onset. This is the most apparent and well-known symptom, though an estimated 30% of patients have little perceptible tremor; these are classified as akinetic-rigid.
- Rigidity: stiffness; increased muscle tone. In combination with a resting tremor, this produces a ratchety, "cogwheel" rigidity when the limb is passively moved.
- bradykinesia/Akinesia: respectively, slowness or absence of movement. Rapid, repetitive movements produce a dysrhythmic and decremental loss of amplitude.
- Postural instability: failure of postural reflexes, which leads to impaired balance and falls.
Other motor symptoms include:
- Gait and posture disturbances:
- Shuffling: gait is characterized by short steps, with feet barely leaving the ground, producing an audible shuffling noise. Small obstacles tend to cause the patient to trip.
- Decreased arm-swing.
- Turning "en bloc": rather than the usual twisting of the neck and trunk and pivoting on the toes, PD patients keep their neck and trunk rigid, requiring multiple small steps to accomplish a turn.
- Stooped, forward-flexed posture. In severe forms, the head and upper shoulders may be bent at a right angle relative to the trunk (camptocormia). [3]
- Festination: a combination of stooped posture, imbalance, and short steps. It leads to a gait that gets progressively faster and faster, often ending in a fall.
- Gait freezing: "freezing" is a manifestation of akinesia (an inability to move). Gait freezing is characterized by an inability to move the feet which may worsen in tight, cluttered spaces or when attempting to initiate gait.
- Dystonia (in about 20% of cases): abnormal, sustained, painful twisting muscle contractions, often affecting the foot and ankle (mainly toe flexion and foot inversion) which often interferes with gait.
- Speech and swallowing disturbances.
- Hypophonia: soft speech. Speech quality tends to be soft, hoarse, and monotonous. Some people with Parkinson's disease claim that their tongue is "heavy" or have cluttered speech.[4]
- Monotonic speech.
- Festinating speech: excessively rapid, soft, poorly-intelligible speech.
- Drooling: most likely caused by a weak, infrequent swallow and stooped posture.
- Dysphagia: impaired ability to swallow. Can lead to aspiration, pneumonia.
- Other motor symptoms:
- Fatigue (up to 50% of cases);
- Masked faces (a mask-like face also known as hypomimia), with infrequent blinking;[5]
- Difficulty rolling in bed or rising from a seated position;
- Micrographia (small, cramped handwriting);
- Impaired fine motor dexterity and motor coordination;
- Impaired gross motor coordination;
- Akathisia, the inability to sit still.
Neuropsychiatric
PD causes cognitive and mood disturbances, being in many cases related.
Estimated prevalence rates of depression vary widely according to the population sampled and methodology used. Reviews of depression estimate its occurrence in anywhere from 20-80% of cases.[6] Estimates from community samples tend to find lower rates than from specialist centres. Most studies use self-report questionnaires such as the Beck Depression Inventory, which may overinflate scores due to physical symptoms. Studies using diagnostic interviews by trained psychiatrists also report lower rates of depression. More generally, there is an increased risk for any individual with depression to go on to develop Parkinson's disease at a later date.[7] Seventy percent of individuals with Parkinson's disease diagnosed with pre-existing depression go on to develop anxiety. Ninety percent of Parkinson's disease patients with pre-existing anxiety subsequently develop depression; apathy or abulia.
Cognitive disturbances include:
- Slowed reaction time; both voluntary and involuntary motor responses are significantly slowed.
- Executive dysfunction, characterized by difficulties in: differential allocation of attention, impulse control, set shifting, prioritizing, evaluating the salience of ambient data, interpreting social cues, and subjective time awareness. This complex is present to some degree in most Parkinson's patients; it may progress to:
- Dementia: a later development in approximately 20-40% of all patients, typically starting with slowing of thought and progressing to difficulties with abstract thought, memory, and behavioral regulation. Hallucinations, delusions and paranoia may develop.
- Short term memory loss; procedural memory is more impaired than declarative memory. Prompting elicits improved recall.
- Non-motor causes of speech/language disturbance in both expressive and receptive language: these include decreased verbal fluency and cognitive disturbance especially related to comprehension of emotional content of speech and of facial expression.[8]
- Medication effects: some of the above cognitive disturbances are improved by dopaminergic medications, while others are actually worsened.[9]
Sleep
- Excessive daytime somnolence
- Initial, intermediate, and terminal insomnia
- Disturbances in REM sleep: disturbingly vivid dreams, and REM Sleep Disorder, characterized by acting out of dream content — can occur years prior to diagnosis
Perception
- Impaired visual contrast sensitivity, spatial reasoning, colour discrimination, convergence insufficiency (characterized by double vision) and oculomotor control
- Dizziness and fainting; usually attributable orthostatic hypotension, a failure of the autonomic nervous system to adjust blood pressure in response to changes in body position
- Impaired proprioception (the awareness of bodily position in three-dimensional space)
- Reduction or loss of sense of smell (hyposmia or anosmia) - can occur years prior to diagnosis
- pain: neuropathic, muscle, joints, and tendons, attributable to tension, dystonia, rigidity, joint stiffness, and injuries associated with attempts at accommodation
Autonomic
- Oily skin and seborrheic dermatitis[10]
- Urinary incontinence, typically in later disease progression
- Nocturia (getting up in the night to pass urine) — up to 60% of cases
- Constipation and gastric dysmotility that is severe enough to endanger comfort and even health
- Altered sexual function: characterized by profound impairment of sexual arousal, behavior, orgasm, and drive is found in mid and late Parkinson disease. Current data addresses male sexual function almost exclusively.
- Weight loss, which is significant over a period of ten years.
Causes
Most people with Parkinson's disease are described as having idiopathic Parkinson's disease (having no specific cause). There are far less common causes of Parkinson's disease including genetic, toxins, head trauma, cerebral anoxia, and drug-induced Parkinson's disease.
Genetic
In recent years, a number of specific genetic mutations causing Parkinson's disease have been discovered, including in certain populations (Contursi, Italy). These account for a small minority of cases of Parkinson's disease. Someone who has Parkinson's disease is more likely to have relatives that also have Parkinson's disease.However, this does not mean that the disorder has been passed on genetically.
Toxins
One theory holds that the disease may result in many or even most cases from the combination of a genetically determined vulnerability to environmental toxins along with exposure to those toxins.[11] This hypothesis is consistent with the fact that Parkinson's disease is not distributed homogeneously throughout the population; its incidence varies geographically. However, it is not consistent with the fact that the first appearance of the syndrome predates the first synthesis of the compounds often attributed to causing Parkinson's disease. The toxins most strongly suspected at present are certain pesticides and transition-series metals such as manganese or iron, especially those that generate reactive oxygen species,[12][13] and/or bind to neuromelanin, as originally suggested by G.C. Cotzias.[14][15] In the Cancer Prevention Study II Nutrition Cohort, a longitudinal investigation, individuals who were exposed to pesticides had a 70% higher incidence of PD than individuals who were not exposed.[16]
The tragedy of a group of drug addicts in California in the early 1980s who consumed a contaminated and illicitly produced batch of the synthetic opiate MPPP brought to light MPTP (pro-toxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyidine) as a specific cause of Parkinson symptoms. This made it possible to develop the first animal model for Parkinson's. MPTP's toxicity likely comes from the generation of reactive oxygen species through tyrosine hydroxylation.[17] The Case of the Frozen Addicts by J. William Langston (Vintage, New York, June 25, 1996) documents this tragedy and describes the first attempts at fetal brain tissue transplants to treat PD.
Other toxin-based models employ PCBs,[18] paraquat[19] (a herbicide) in combination with maneb (a fungicide),[20] rotenone[21] (an insecticide), and specific organochlorine pesticides including dieldrin[22] and lindane.[23] Rotenone is an inhibitor of complex 1 of the electron transport chain. It easily crosses membranes due to its extremely hydrophobic properties. Rotenone, therefore, does not rely on the dopamine transporter to enter into the cytoplasm. Numerous studies have found an increase in PD in persons who consume rural well water; researchers theorize that water consumption is a proxy measure of pesticide exposure. In agreement with this hypothesis are studies which have found a dose-dependent increase in PD in persons exposed to agricultural chemicals.
Head trauma
Past episodes of head trauma are reported more frequently by individuals with Parkinson's disease than by others in the population.[24][25][26] A recent methodologically strong retrospective study[24] found that those who have experienced a head injury are four times more likely to develop Parkinson’s disease than those who have never suffered a head injury. The risk of developing Parkinson’s increases eightfold for patients who have had head trauma requiring hospitalization, and it increases 11-fold for patients who had experienced severe head injury. The authors comment that since head trauma is a rare event, the contribution to PD incidence is slight. They express further concern that their results may be biased by recall, i.e., the PD patients because they reflect upon the causes of their illness, may remember head trauma better than the non-ill control subjects. These limitations were overcome recently by Tanner and colleagues,[27] who found a similar risk of 3.8, with increasing risk associated with more severe injury and hospitalization. However, whether the head trauma actually contributed to Parkinson's disease development or the early symptoms of clumsiness associated with Parkinson's causes individuals to have more head trauma is still unknown.
Pathophysiology
Dopaminergic pathways of the human brain in normal condition (left) and Parkinson's disease (right). Red Arrows indicate suppression of the target, blue arrows indicate stimulation of target structure.
The symptoms of Parkinson's disease result from the loss of pigmented dopamine-secreting (dopaminergic) cells in the pars compacta region of the substantia nigra (literally "black substance"). These neurons project to the striatum and their loss leads to alterations in the activity of the neural circuits within the basal ganglia that regulate movement, in essence an inhibition of the direct pathway and excitation of the indirect pathway.
The direct pathway facilitates movement and the indirect pathway inhibits movement, thus the loss of these cells leads to a hypokinetic movement disorder. The lack of dopamine results in increased inhibition of the ventral anterior nucleus of the thalamus, which sends excitatory projections to the motor cortex, thus leading to hypokinesia.
There are four major dopamine pathways in the brain; the nigrostriatal pathway, referred to above, mediates movement and is the most conspicuously affected in early Parkinson's disease. The other pathways are the mesocortical, the mesolimbic, and the tuberoinfundibular. Disruption of dopamine along the non-striatal pathways likely explains much of the neuropsychiatric pathology associated with Parkinson's disease.
The mechanism by which the brain cells in Parkinson's are lost may consist of an abnormal accumulation of the protein alpha-synuclein bound to ubiquitin in the damaged cells. The alpha-synuclein-ubiquitin complex cannot be directed to the proteosome. This protein accumulation forms proteinaceous cytoplasmic inclusions called Lewy bodies. The latest research on pathogenesis of disease has shown that the death of dopaminergic neurons by alpha-synuclein is due to a defect in the machinery that transports proteins between two major cellular organelles — the endoplasmic reticulum (ER) and the Golgi apparatus. Certain proteins like Rab1 may reverse this defect caused by alpha-synuclein in animal models.[28]
Excessive accumulations of iron, which are toxic to nerve cells, are also typically observed in conjunction with the protein inclusions. Iron and other transition metals such as copper bind to neuromelanin in the affected neurons of the substantia nigra. Neuromelanin may be acting as a protective agent. The most likely mechanism is generation of reactive oxygen species.[12] Iron also induces aggregation of synuclein by oxidative mechanisms.[29] Similarly, dopamine and the byproducts of dopamine production enhance alpha-synuclein aggregation. The precise mechanism whereby such aggregates of alpha-synuclein damage the cells is not known. The aggregates may be merely a normal reaction by the cells as part of their effort to correct a different, as-yet unknown, insult. Based on this mechanistic hypothesis, a transgenic mouse model of Parkinson's has been generated by introduction of human wild-type alpha-synuclein into the mouse genome under control of the platelet-derived-growth factor-β promoter.[30]
Diagnosis
18F PET scan shows decreased dopamine activity in the basal ganglia, a pattern which aids in diagnosing Parkinson's disease.
Typically, the diagnosis is based on medical history and neurological examination conducted by interviewing and observing the patient in person using the Unified Parkinson's Disease Rating Scale. A radiotracer for SPECT scanning machines called DaTSCAN and made by General Electric is specialized for diagnosing Parkinson's Disease, but it is only marketed in Europe. Due to this, the disease can be difficult to diagnose accurately, especially in its early stages. Due to symptom overlap with other diseases, only 75% of clinical diagnoses of PD are confirmed to be idiopathic PD at autopsy.[31] Early signs and symptoms of PD may sometimes be dismissed as the effects of normal aging. The physician may need to observe the person for some time until it is apparent that the symptoms are consistently present. Usually doctors look for shuffling of feet and lack of swing in the arms. Doctors may sometimes request brain scans or laboratory tests in order to rule out other diseases. However, CT and MRI brain scans of people with PD usually appear normal.
Clinical practice guidelines introduced in the UK in 2006 state that the diagnosis and follow-up of Parkinson's disease should be done by a specialist in the disease, usually a neurologist or geriatrician with an interest in movement disorders.[2]
Treatment
Parkinson's disease is a chronic disorder that requires broad-based management including patient and family education, support group services, general wellness maintenance, physiotherapy, exercise, and nutrition.[2] At present, there is no cure for PD, but medications or surgery can provide relief from the symptoms.
Levodopa
Stalevo for treatment of Parkinson's disease
The most widely used form of treatment is L-dopa in various forms. L-dopa is transformed into dopamine in the dopaminergic neurons by L-aromatic amino acid decarboxylase (often known by its former name dopa-decarboxylase). However, only 1-5% of L-DOPA enters the dopaminergic neurons. The remaining L-DOPA is often metabolised to dopamine elsewhere, causing a wide variety of side effects. Due to feedback inhibition, L-dopa results in a reduction in the endogenous formation of L-dopa, and so eventually becomes counterproductive.
Carbidopa and benserazide are dopa decarboxylase inhibitors. They help to prevent the metabolism of L-dopa before it reaches the dopaminergic neurons and are generally given as combination preparations of carbidopa/levodopa (co-careldopa) (e.g. Sinemet, Parcopa) and benserazide/levodopa (co-beneldopa) (e.g. Madopar). There are also controlled release versions of Sinemet and Madopar that spread out the effect of the L-dopa. Duodopa is a combination of levodopa and carbidopa, dispersed as a viscous gel. Using a patient-operated portable pump, the drug is continuously delivered via a tube directly into the upper small intestine, where it is rapidly absorbed. There is also Stalevo (Carbidopa, Levodopa and Entacapone).
COMT Inhibitors
Tolcapone inhibits the COMT enzyme, thereby prolonging the effects of L-dopa, and so has been used to complement L-dopa. However, due to its possible side effects such as liver failure, it's limited in its availability. A similar drug, entacapone has not been shown to cause significant alterations of liver function and maintains adequate inhibition of COMT over time.[32]
Dopamine agonists
The dopamine agonists bromocriptine, pergolide, pramipexole, ropinirole , cabergoline, apomorphine, and lisuride are moderately effective. These have their own side effects including those listed above in addition to somnolence, hallucinations and/or insomnia. Several forms of dopamine agonism have been linked with a markedly increased risk of problem gambling. Dopamine agonists initially act by stimulating some of the dopamine receptors. However, they cause the dopamine receptors to become progressively less sensitive, thereby eventually increasing the symptoms.
Dopamine agonists can be useful for patients experiencing on-off fluctuations and dyskinesias as a result of high doses of L-dopa. Apomorphine can be administered via subcutaneous injection using a small pump which is carried by the patient. A low dose is automatically administered throughout the day, reducing the fluctuations of motor symptoms by providing a steady dose of dopaminergic stimulation. After an initial "apomorphine challenge" in hospital to test its effectiveness and brief patient and primary caregiver (often a spouse or partner), the latter of whom takes over maintenance of the pump. The injection site must be changed daily and rotated around the body to avoid the formation of nodules. Apomorphine is also available in a more acute dose as an autoinjector pen for emergency doses such as after a fall or first thing in the morning. Nausea and vomiting are common, and may require domperidone (an antiemetic).
MAO-B inhibitors
Selegiline and rasagiline reduce the symptoms by inhibiting monoamine oxidase-B (MAO-B), which inhibits the breakdown of dopamine secreted by the dopaminergic neurons. Metabolites of selegiline include L-amphetamine and L-methamphetamine (not to be confused with the more notorious and potent dextrorotary isomers). This might result in side effects such as insomnia. Use of L-dopa in conjunction with selegiline has increased mortality rates that have not been effectively explained. Another side effect of the combination can be stomatitis. One report raised concern about increased mortality when MAO-B inhibitors were combined with L-dopa;[33] however subsequent studies have not confirmed this finding.[34] Unlike other non selective monoamine oxidase inhibitors, tyramine-containing foods do not cause a hypertensive crisis.
Surgery and deep brain stimulation
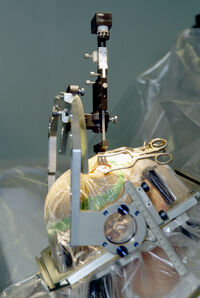
Illustration showing an electrode placed deep seated in the brain
Treating Parkinson's disease with surgery was once a common practice, but after the discovery of levodopa, surgery was restricted to only a few cases. Studies in the past few decades have led to great improvements in surgical techniques, and surgery is again being used in people with advanced PD for whom drug therapy is no longer sufficient.
Deep brain stimulation is presently the most used surgical means of treatment, but other surgical therapies that have shown promise include surgical lesion of the subthalamic nucleus[35] and of the internal segment of the globus pallidus, a procedure known as pallidotomy.[36]
Neurorehabilitation
There is partial evidence that speech or mobility problems can improve with rehabilitation although studies are still scarce and of low quality.[37][38][39][40] Regular physical exercise and/or therapy can be beneficial to the patient for maintaining and improving mobility, flexibility, strength, gait speed, and quality of life;[39] and speech therapy may improve voice and speech function.[40] One of the most widely practiced treatment for the speech disorders associated with Parkinson's disease is the Lee Silverman Voice Treatment (LSVT). LSVT focuses on increasing vocal loudness.[41]
Prognosis
PD is not considered to be a fatal disease by itself, but it progresses with time. The average life expectancy of a PD patient is generally lower than for people who do not have the disease.[42] In the late stages of the disease, PD may cause complications such as choking, pneumonia, and falls that can lead to death.
The progression of symptoms in PD may take 20 years or more. In some people, however, the disease progresses more quickly. There is no way to predict what course the disease will take for an individual person. With appropriate treatment, most people with PD can live productive lives for many years after diagnosis.
In at least some studies, it has been observed that mortality was significantly increased, and longevity decreased among nursing home patients as compared to community dwelling patients.[43]
One commonly used system for describing how the symptoms of PD progress is called the Hoehn and Yahr scale. Another commonly used scale is the Unified Parkinson's Disease Rating Scale (UPDRS). This much more complicated scale has multiple ratings that measure motor function, and also mental functioning, behavior, mood, and activities of daily living. Both the Hoehn and Yahr scale and the UPDRS are used to measure how individuals are faring and how much treatments are helping them. It should be noted that neither scale is specific to Parkinson's disease; that patients with other illnesses can score in the Parkinson's range.
Epidemiology
According to some sources, Parkinsons disease is slightly less prevalent in the African-American community. The average crude prevalence is estimated at being from 120-180 out of 100,000 among the Caucasian (white) community.[44] The Parsi community in Mumbai, India suffers from particularly high rates of Parkinson's disease.[45][46]
History
Symptoms of Parkinson's disease have been known and treated since medieval times, most notably by Averroes.[47] However, it was not formally recognized and its symptoms were not documented until 1817 in An Essay on the Shaking Palsy[48] by the British physician James Parkinson. Parkinson's disease was then known as paralysis agitans, the term "Parkinson's disease" being coined later by Jean-Martin Charcot. The underlying biochemical changes in the brain were identified in the 1950s due largely to the work of Swedish scientist Arvid Carlsson, who later went on to win a Nobel Prize. L-dopa entered clinical practice in 1967,[49] and the first large study reporting improvements in patients with Parkinson's disease resulting from treatment with L-dopa was published in 1968.[50]
Research directions
Gene therapy
Currently under investigation is gene therapy. This involves using a non-infectious virus to shuttle a gene into a part of the brain called the subthalamic nucleus (STN). The gene used leads to the production of an enzyme called glutamic acid decarboxylase (GAD), which catalyses the production of a neurotransmitter called GABA.[51] GABA acts as a direct inhibitor on the overactive cells in the STN.
GDNF infusion involves the infusion of GDNF (glial-derived neurotrophic factor) into the basal ganglia using surgically implanted catheters. Via a series of biochemical reactions, GDNF stimulates the formation of L-dopa. GDNF therapy is still in development.
Implantation of stem cells genetically engineered to produce dopamine or stem cells that transform into dopamine-producing cells has already started being used. These could not constitute cures because they do not address the considerable loss of activity of the dopaminergic neurons. Initial results have been unsatisfactory, with patients still retaining their drugs and symptoms.
Neuroprotective treatments
Neuroprotective treatments are at the forefront of PD research, but are still under clinical scrutiny.[52] These agents could protect neurons from cell death induced by disease presence resulting in a slower progression of disease. Agents currently under investigation as neuroprotective agents include anti-apoptotic drugs (CEP 1347 and CTCT346), lazaroids, bioenergetics, antiglutamatergic agents and dopamine receptors.[53] Clinically evaluated neuroprotective agents are the monoamine oxidase inhibitors selegiline[54] and rasagiline, dopamine agonists, and the complex I mitochondrial fortifier coenzyme Q10.
Neural transplantation
The first prospective randomised double-blind sham-placebo controlled trial of dopamine-producing cell transplants failed to show an improvement in quality of life although some significant clinical improvements were seen in patients below the age of 60.[55] A significant problem was the excess release of dopamine by the transplanted tissue, leading to dystonias.[56] Research in African green monkeys suggests that the use of stem cells might in future provide a similar benefit without inducing dystonias.[57]
Alternative Treatments
Nutrients have been used in clinical studies and are used by people with PD in order to partially treat PD or slow down its deterioration. The L-dopa precursor L-tyrosine was shown to relieve an average of 70% of symptoms.[58] Ferrous iron, the essential cofactor for L-dopa biosynthesis was shown to relieve between 10% and 60% of symptoms in 110 out of 110 patients.[59] [60] More limited efficacy has been obtained with the use of THFA, NADH, and pyridoxine—coenzymes and coenzyme precursors involved in dopamine biosynthesis.[61] Vitamin C and vitamin E in large doses are commonly used by patients in order to theoretically lessen the cell damage that occurs in PD. This is because the enzymes superoxide dismutase and catalase require these vitamins in order to nullify the superoxide anion, a toxin commonly produced in damaged cells. However, in the randomized controlled trial, DATATOP of patients with early PD, no beneficial effect for vitamin E compared to placebo was seen.[54] Coenzyme Q10 has more recently been used for similar reasons. MitoQ is a newly developed synthetic substance that is similar in structure and function to coenzyme Q10.
Studies looking at qigong in PD have not reached consensus on its efficacy.[62][63]
Mucuna pruriens, is a natural source of therapeutic quantities of L-dopa, and has been under some investigation.[64]
See also
References
- ↑ 1.0 1.1 1.2 Jankovic J (April 2008). Parkinson's disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatr. 79 (4): 368–76.
- ↑ 2.0 2.1 2.2 Template:NICE
- ↑ Lepoutre A, Devos D, Blanchard-Dauphin A, et al (2006). A specific clinical pattern of camptocormia in Parkinson's disease. J. Neurol. Neurosurg. Psychiatr. 77 (11): 1229–34.
- ↑ Fox, Michael (2003). Lucky Man: A Memoir, 214, Hyperion.
- ↑ Deuschl G, Goddemeier C (1998). Spontaneous and reflex activity of facial muscles in dystonia, Parkinson's disease, and in normal subjects. J. Neurol. Neurosurg. Psychiatr. 64 (3): 320–4.
- ↑ Lieberman A (2006). Depression in Parkinson's disease -- a review. Acta Neurol Scand 113 (1): 1–8.
- ↑ Ishihara L, Brayne C (2006). A systematic review of depression and mental illness preceding Parkinson's disease. Acta Neurol Scand 113 (4): 211–20.
- ↑ Pell M (1996). On the receptive prosodic loss in Parkinson's disease. Cortex 32 (4): 693–704.
- ↑ Frank MJ (2005). Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal of cognitive neuroscience 17 (1): 51–72.
- ↑ Gupta A, Bluhm R (2004). Seborrheic dermatitis. Journal of the European Academy of Dermatology and Venereology : JEADV 18 (1): 13–26; quiz 19–20.
- ↑ Di Monte DA, Lavasani M, Manning-Bog AB (2002). Environmental factors in Parkinson's disease. Neurotoxicology 23 (4-5): 487–502.
- ↑ 12.0 12.1 Jenner P (1998). Oxidative mechanisms in nigral cell death in Parkinson's disease. Mov. Disord. 13 Suppl 1: 24–34. Cite error: Invalid
<ref>tag; name "Jenner1998" defined multiple times with different content - ↑ Chiueh CC, Andoh T, Lai AR, Lai E, Krishna G (2000). Neuroprotective strategies in Parkinson's disease: protection against progressive nigral damage induced by free radicals. Neurotoxicity research 2 (2-3): 293–310.
- ↑ Cotzias G (1966). Manganese, melanins and the extrapyramidal system. J Neurosurg 24 (1): Suppl:170–80.
- ↑ Barbeau A (1984). Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias). Neurotoxicology 5 (1): 13–35.
- ↑ Ascherio A, Chen H, Weisskopf M, et al (2006). Pesticide exposure and risk for Parkinson's disease. Ann Neurol 60 (2): 197–203.
- ↑ Chiueh C, Wu R, Mohanakumar K, Sternberger L, Krishna G, Obata T, Murphy D (1994). In vivo generation of hydroxyl radicals and MPTP-induced dopaminergic toxicity in the basal ganglia. Ann N Y Acad Sci 738: 25–36.
- ↑ includeonly>Orr, Leslie. "PCBs, fungicide open brain cells to Parkinson's assault", Medical News Today, February 10, 2005.
- ↑ Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA (2002). The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J. Biol. Chem. 277 (3): 1641–4.
- ↑ Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA (2000). The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J. Neurosci. 20 (24): 9207–14.
- ↑ Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000). Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3 (12): 1301–6.
- ↑ Kitazawa M, Anantharam V, Kanthasamy AG (2001). Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic. Biol. Med. 31 (11): 1473–85.
- ↑ Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D (2000). Organochlorine insecticides in substantia nigra in Parkinson's disease. J. Toxicol. Environ. Health Part A 59 (4): 229–34.
- ↑ 24.0 24.1 Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA (2003). Head trauma preceding PD: a case-control study. Neurology 60 (10): 1610–5.
- ↑ Stern M, Dulaney E, Gruber SB, et al (1991). The epidemiology of Parkinson's disease. A case-control study of young-onset and old-onset patients. Arch. Neurol. 48 (9): 903–7.
- ↑ Uryu K, Giasson BI, Longhi L, et al (2003). Age-dependent synuclein pathology following traumatic brain injury in mice. Exp. Neurol. 184 (1): 214–24.
- ↑ Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW (2006). Head injury and Parkinson's disease risk in twins. Ann. Neurol. 60 (1): 65–72.
- ↑ "Parkinson's Disease Mechanism Discovered," HHMI Research News June 22, 2006.
- ↑ Kaur D, Andersen J (2002). Ironing out Parkinson's disease: is therapeutic treatment with iron chelators a real possibility?. Aging Cell 1 (1): 17–21.
- ↑ Masliah E, Rockenstein E, Veinbergs I, et al (2000). Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287 (5456): 1265–9.
- ↑ Gelb D, Oliver E, Gilman S (1999). Diagnostic criteria for Parkinson disease. Arch Neurol 56 (1): 33–9.
- ↑ R. Cilia et al. (2006). Long-term Efficacy of Entacapone in Patients with Parkinson's Disease and Motor Fluctuations - A Six-Year Clinical Follow-Up Study.
- ↑ Thorogood M, Armstrong B, Nichols T, Hollowell J (1998). Mortality in people taking selegiline: observational study. BMJ 317 (7153): 252–4.
- ↑ Marras C, McDermott M, Rochon P, Tanner C, Naglie G, Rudolph A, Lang A (2005). Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology 64 (1): 87–93.
- ↑ Guridi J, Obeso JA (2001). The subthalamic nucleus, hemiballismus and Parkinson's disease: reappraisal of a neurosurgical dogma. Brain 124 (Pt 1): 5–19.
- ↑ Fukuda M, Kameyama S, Yoshino M, Tanaka R, Narabayashi H (2000). Neuropsychological outcome following pallidotomy and thalamotomy for Parkinson's disease. Stereotactic and functional neurosurgery 74 (1): 11–20.
- ↑ Deane KH, Jones D, Playford ED, Ben-Shlomo Y, Clarke CE (2001). Physiotherapy for patients with Parkinson's Disease: a comparison of techniques. Cochrane Database Syst Rev (3): CD002817.
- ↑ Deane KH, Whurr R, Playford ED, Ben-Shlomo Y, Clarke CE (2001). A comparison of speech and language therapy techniques for dysarthria in Parkinson's disease. Cochrane Database Syst Rev (2): CD002814.
- ↑ 39.0 39.1 Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL (April 2008). The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 23 (5): 631–40.
- ↑ 40.0 40.1 Schulz GM (July 2002). The effects of speech therapy and pharmacological treatments on voice and speech in Parkinson s disease: a review of the literature. Curr. Med. Chem. 9 (14): 1359–66.
- ↑ Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG (November 2006). The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Semin Speech Lang 27 (4): 283–99.
- ↑ Parkinson's Disease. Mayo Clinic: College of Medicine. URL accessed on 2006-11-04.
- ↑ Goetz CG, Stebbins GT (1995). Mortality and hallucinations in nursing home patients with advanced Parkinson's disease. Neurology 45 (4): 669–71.
- ↑ http://holisticonline.com/Remedies/Parkinson/pd_who-gets.htm
- ↑ http://archneur.ama-assn.org/cgi/content/abstract/45/12/1321
- ↑ http://holisticonline.com/Remedies/Parkinson/pd_who-gets.htm
- ↑ Manyam BV, Sánchez-Ramos JR (1999). Traditional and complementary therapies in Parkinson's disease. Advances in neurology 80: 565–74.
- ↑ Parkinson J (2002). An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 14 (2): 223–36; discussion 222.
- ↑ Hornykiewicz O (2002). L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent. Amino Acids 23 (1-3): 65–70.
- ↑ Cotzias G (1968). L-Dopa for Parkinsonism. N Engl J Med 278 (11): 630.
- ↑ Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ (2007). Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369 (9579): 2097–105.
- ↑ Bonuccelli U, Del Dotto P (2006). New pharmacologic horizons in the treatment of Parkinson disease. Neurology 67 (2): 30–38.
- ↑ Djaldetti R, Melamed E (2002). New drugs in the future treatment of Parkinson's disease. J. Neurol. 249 Suppl 2: II30–5.
- ↑ 54.0 54.1 (1993) Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N. Engl. J. Med. 328 (3): 176–83.
- ↑ Freed CR, Greene PE, Breeze RE, et al (2001). Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 344 (10): 710–9.
- ↑ Redmond DE (2002). Cellular replacement therapy for Parkinson's disease--where we are today?. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 8 (5): 457–88.
- ↑ Redmond E et al (2007). Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proceedings of the National Academy of Sciences 104 (29): 12175.
- ↑ Lemoine P, Robelin N, Sebert P, Mouret J (1986). La L-tyrosine : traitement au long cours de la maladie de Parkinson [L-tyrosine : A long term treatment of Parkinson's Disease]. Comptes rendus academie des sciences 309: 43–47.
- ↑ Birkmayer W, Birkmayer JG (1986). Iron, a new aid in the treatment of Parkinson patients. J. Neural Transm. 67 (3-4): 287–92.
- ↑ (1989) Editors Przuntek H , Riederer P Early diagnosis and preventive therapy in Parkinson's disease, p. 323, Springer.
- ↑ Dopamine biosynthesis. (Word doc) University of Chicago Personal Web Pages. URL accessed on 2006-11-04.
- ↑ Schmitz-Hubsch T (2006). Qigong exercise for the symptoms of Parkinson's disease: a randomized, controlled pilot study. Mov Disord 21 (4): 543–548.
- ↑ Burini D, Farabollini B, Iacucci S, et al (2006). A randomised controlled cross-over trial of aerobic training versus qigong in advanced Parkinson's disease. Europa medicophysica 42 (3): 231–8.
- ↑ Katzenschlager R, Evans A, Manson A, et al (2004). Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study. J. Neurol. Neurosurg. Psychiatr. 75 (12): 1672–7.
External links
- Parkinson's Disease at the Open Directory Project
- Parkinson's Disease: Hope Through Research (National Institute of Neurological Disorders and Stroke)
- World Parkinson Disease Association
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |

