No edit summary |
(update wp) |
||
| Line 1: | Line 1: | ||
{{BioPsy}} |
{{BioPsy}} |
||
| − | '''Neural oscillation''' is rhythmic or repetitive neural activity in the [[central nervous system]]. Neural tissue can generate oscillatory activity in many ways, driven either by mechanisms localized within individual [[neurons]] or by |
+ | '''Neural oscillation''' is rhythmic or repetitive neural activity in the [[central nervous system]]. Neural tissue can generate [[oscillations|oscillatory activity]] in many ways, driven either by mechanisms localized within individual [[neurons]] or by interactions between neurons. In individual neurons, oscillations can appear either as oscillations in [[membrane potential]] or as rhythmic patterns of [[action potentials]], which then produce oscillatory activation of [[post-synaptic potential|post-synaptic]] neurons. At the level of [[neural ensemble]]s, synchronized activity of large numbers of neurons can give rise to [[macroscopic scale|macroscopic]] oscillations, which can be observed in the [[electroencephalography|electroencephalogram]] (EEG). Oscillatory activity in groups of neurons generally arise from feedback connections between the neurons that result in the synchronization of their firing patterns. The interaction between neurons can give rise to oscillations at a different frequency than the firing frequency of individual neurons. A well-known example of macroscopic neural oscillations is [[alpha wave|alpha activity]]. |
| + | Neural oscillations were observed by researchers as early as [[Hans Berger]], but their functional role is still not fully understood. The possible roles of neural oscillations include [[neural binding|feature binding]], [[neural code|information transfer mechanisms]] and the [[central pattern generator|generation of rhythmic motor output]]. Over the last decades more insight has been gained, especially with advances in [[brain imaging]]. A major area of research in neuroscience involves determining how oscillations are generated and what their roles are. Oscillatory activity in the brain is widely observed at different [[levels of Organization (anatomy)|levels of observation]] and is thought to play a key role in processing neural information. Numerous experimental studies indeed support a functional role of neural oscillations; a unified interpretation, however, is still lacking. |
||
| − | [[Image:SimulationNeuralOscillations.png|thumb|right|400px|Simulation of neural oscillations at 10 [[Hertz|Hz]]. Upper panel shows spiking of individual neurons (with each dot representing an individual [[action potential]] within the population of neurons), and the lower panel the [[local field potential]] reflecting their summed activity.]] |
||
| + | |||
| + | [[File:SimulationNeuralOscillations.png|thumb|right|400px|Simulation of neural oscillations at 10 [[Hertz|Hz]]. Upper panel shows spiking of individual neurons (with each dot representing an individual [[action potential]] within the population of neurons), and the lower panel the [[local field potential]] reflecting their summed activity. Figure illustrates how synchronized patterns of action potentials may result in macroscopic oscillations that can be measured outside the scalp.]] |
||
| + | |||
| + | {{TOC limit|limit=3}} |
||
== Overview == |
== Overview == |
||
| + | Neural oscillations are observed throughout the central nervous system and at all levels, e.g., [[action potential|spike trains]], [[local field potentials]] and large-scale oscillations which can be measured by [[electroencephalography]]. In general, oscillations can be characterized by their [[frequency]], [[amplitude]] and [[Phase (waves)|phase]]. These signal properties can be extracted from neural recordings using [[time-frequency analysis]]. In large-scale oscillations, amplitude changes are considered to result from changes in synchronization within a [[neural ensemble]], also referred to as local synchronization. In addition to local synchronization, oscillatory activity of distant neural structures (single neurons or neural ensembles) can synchronize. Neural oscillations and synchronization have been linked to many cognitive functions such as information transfer, perception, motor control and memory.<ref name = "Fries 2001">{{cite journal | author = Fries P | title = A mechanism for cognitive dynamics: neuronal communication through neuronal coherence | journal = TICS | volume = 9 | pages = 474–480 | year = 2001}}</ref><ref>{{cite journal | author = Fell J, Axmacher N | title = The role of phase synchronization in memory processes | journal = Nat Rev Neurosci | volume = 12 | pages = 105–118 | year = 2011}}</ref><ref name = "Schnitzler 2005">{{cite journal | doi = 10.1038/nrn1650 | author = Schnitzler A, Gross J | title = Normal and pathological oscillatory communication in the brain | journal = Nat Rev Neurosci | volume = 6 | pages = 285–296 | year = 2005 | pmid=15803160 | issue=4}}</ref> |
||
| − | EEG signals oscillate across a spectrum of frequencies. Scientists have constructed an arbitrary set of frequency bands which group specific ranges of frequencies from this spectrum. The first discovered and best-known frequency band is [[alpha waves|alpha activity]] (8–12 Hz).<ref>{{cite journal | author = Berger H | title = Uber das Elektroenkephalogramm des Menschen | journal = Arch |
||
| + | |||
| − | Psychiat Nervenkr | volume = 87 | pages = 527–570 | year = 1929 | doi = 10.1007/BF01797193}}</ref> Other frequency bands are: [[delta wave|delta]] (1–4 Hz), [[theta wave|theta]] (4–8 Hz), [[beta wave|beta]] (13–30 Hz) and [[gamma wave|gamma]] (30–70 Hz) frequency band. Although neural oscillations in human brain activity are mostly investigated using EEG recordings, they are also observed in animals using more invasive recording techniques such as single-unit recordings. Intracellularly, oscillations are observed in [[subthreshold membrane potential oscillations]],<ref name = "Llinas 1986">{{cite journal | author = Llinas R, Yarom Y | year = 1986 | title = Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study | journal = J Physiol | volume=376 | pages=163–182}}</ref> whereas extracellularly they are reflected in changes in [[local field potentials]] (LFPs). Large-scale oscillations that are observable outside the scalp with EEG or [[magnetoencephalography|MEG]] arise through synchronous activity of large numbers of neurons. |
||
| + | Neural oscillations have been most widely studied in neural activity generated by large groups of neurons. Large-scale activity can measured by techniques such as electroencephalography (EEG). In general, EEG signals have a broad spectral content similar to [[pink noise]], but also reveal oscillatory activity in specific frequency bands. The first discovered and best-known frequency band is [[alpha waves|alpha activity]] (8–12 Hz) that can be detected from the [[occipital lobe]] during relaxed wakefulness and increases when the eyes are closed.<ref>{{cite journal | author = Berger H | last2 = Gray | first2 = CM | title = Uber das Elektroenkephalogramm des Menschen | journal = Arch Psychiat Nervenkr | volume = 87 | pages = 527–570 | year = 1929 | doi = 10.1007/BF01797193 }}</ref> Other frequency bands are: [[delta wave|delta]] (1–4 Hz), [[theta wave|theta]] (4–8 Hz), [[beta wave|beta]] (13–30 Hz) and [[gamma wave|gamma]] (30–70 Hz) frequency band, where faster rhythms such as gamma activity have been linked to cognitive processing. Indeed, EEG signals change dramatically during [[sleep]] and show a transition from faster frequencies such as alpha waves to increasingly slower frequencies. In fact, different sleep stages are commonly characterized by their spectral content.<ref>{{cite journal |author=Dement W, Kleitman N |title=Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming |journal=Electroencephalogr Clin Neurophysiol |year=1957 |volume=9 |pages=673–90 |doi=10.1016/0013-4694(57)90088-3 |pmid=13480240 |issue=4}}</ref> Consequently, neural oscillations have been linked to cognitive states, such as [[awareness]] and [[consciousness]].<ref>{{cite journal |author=Engel AK, Singer W |title=Temporal binding and the neural correlates of sensory awareness |year=2001 |volume=5 |pages=16–25 |doi=10.1016/S1364-6613(00)01568-0 |issue=1 |pmid=11164732}}</ref><ref name = "Varela 2001">{{cite journal | author = Varela F, Lachaux JP, Rodriguez E, Martinerie J | title = The brainweb: phase synchronization and large-scale integration | journal = Nat Rev Neurosci | volume = 2 | pages = 229–239 | year = 2001 | doi = 10.1038/35067550 | pmid = 11283746 | issue = 4}}</ref> |
||
| + | |||
| + | Although neural oscillations in human brain activity are mostly investigated using EEG recordings, they are also observed using more invasive recording techniques such as [[single-unit recording]]s. Neurons can generate rhythmic patterns of [[action potential]]s or spikes. Some types of neurons have the tendency to fire at particular frequencies, so-called ''resonators''.<ref name = "Izhikevich 2007">{{cite book | author = Izhikevich EM | title = Dynamical systems in neuroscience | publisher = The MIT Press | location = Cambridge, Massachusetts | year = 2007}}</ref> [[Bursting]] is another form of rhythmic spiking. Spiking patterns are considered fundamental for [[neural coding|information coding]] in the brain. Oscillatory activity can also be observed in the form of [[subthreshold membrane potential oscillations]] (i.e. in the absence of action potentials).<ref name = "Llinas 1986">{{cite journal | author = Llinas R, Yarom Y | year = 1986 | title = Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study | journal = J Physiol | volume=376 | pages=163–182 | pmid=3795074}}</ref> If numerous neuron spike in [[synchronization|synchrony]], they can give rise to oscillations in [[local field potentials]] (LFPs). Quantitative models can estimate the strength of neural oscillation in recorded data.<ref>{{cite journal | author =Mureşan RC, Jurjuţ OF, Moca VV, Singer W, Nikolić D | title = The Oscillation Score: An Efficient Method for Estimating Oscillation Strength in Neuronal Activity | journal = Journal of Neurophysiology | year = 2008 | volume = 99 |issue=3 | pages = 1333–1353 }}</ref> |
||
| + | |||
| + | Neural oscillations are commonly studied from a mathematical framework and belong to the field of “neurodynamics”, an area of research in the [[cognitive science]]s that places a strong focus upon the dynamic character of neural activity in describing [[brain]] function.<ref>{{cite journal | doi = 10.1086/286819 | author = Burrow T | title = The neurodynamics of behavior. A phylobiological foreword | journal = Philosophy of Science | volume = 10 | pages = 271–288 | year = 1943}}</ref>. It considers the brain a [[dynamical system]] and uses [[differential equations]] to describe how neural activity evolves over time. In particular, it aims to relate dynamic patterns of brain activity to cognitive functions such as perception and memory. In very [[abstract structure|abstract form]], neural oscillations can be analyzed [[Analytical expression|analytically]]. When studied in a more physiologically realistic setting, oscillatory activity is generally studied using [[computer simulation]]s of a [[computational neuroscience|computational model]]. |
||
| + | |||
| + | The functions of neural oscillations are wide ranging and vary for different types of oscillatory activity. Examples are the generation of rhythmic activity such as a [[Cardiac cycle|heartbeat]] and the [[neural binding]] of sensory features in perception, such as the shape and color of an object. Neural oscillations also play an important role in many [[neurological disorder]]s, such as excessive synchronization during [[seizure]] activity in [[epilepsy]] or [[tremor]] in patients with [[Parkinson's disease]]. Oscillatory activity can also be used to control external devises in [[brain-computer interface]]s, in which subjects can control an external device by changing the amplitude of particular brain rhythmics. |
||
| + | |||
| + | ==Physiology== |
||
| + | {{main|Electrophysiology}} |
||
| + | |||
| + | Oscillatory activity is observed throughout the [[central nervous system]] at all levels of organization. Three different levels have been widely recognized: the micro-scale (activity of a single neuron), the meso-scale (activity of a local group of neurons) and the macro-scale (activity of different brain regions).<ref name = "Haken 1996">{{cite book | author=Haken H | title= Principles of brain functioning | year=1996 | publisher=Springer | isbn=3-540-58967-8}}</ref> |
||
| + | [[File:Current Clamp recording of Neuron.GIF|thumb|right|[[Tonic (physiology)|Tonic]] firing pattern of single neuron showing rhythmic spiking activity]] |
||
| + | |||
| + | === Microscopic === |
||
| + | |||
| + | Neurons generate [[action potentials]] resulting from changes in the electric membrane potential. Neurons can generate multiple action potentials in sequence forming so-called spike trains. These spike trains are the basis for [[neural coding]] and information transfer in the brain. Spike trains can form all kinds of patterns, such as rhythmic spiking and [[bursting]], and often display oscillatory activity.<ref name = "Wang 2010">{{cite journal | author=Wang XJ | title= Neurophysiological and computational principles of cortical rhythms in cognition | year=2010 | journal=Physiol Rev | volume=90 | pages = 1195–1268 | doi=10.1152/physrev.00035.2008}}</ref> Oscillatory activity in single neurons can also be observed in [[subthreshold membrane potential oscillations|sub-threshold fluctuations]] in membrane potential. These rhythmic changes in membrane potential do not reach the critical threshold and therefore do not result in an action potential. They can result from postsynaptic potentials from synchronous inputs or from intrinsic properties of neurons. |
||
| + | |||
| + | Neuronal spiking can be classified by their activity patterns. The excitability of neurons can be subdivided in Class I and II. Class I neurons can generate action potentials with arbitrarily low frequency depending on the input strength, whereas Class II neurons generate action potentials in a certain frequency band, which is relatively insensitive to changes in input strength.<ref name = "Izhikevich 2007"/> Class II neurons are also more prone to display sub-threshold oscillations in membrane potential. |
||
| + | |||
| + | === Mesoscopic === |
||
| + | |||
| + | A group of neurons can also generate oscillatory activity. Through synaptic interactions the firing patterns of different neurons may become synchronized and the rhythmic changes in electric potential caused by their action potentials will add up ([[Interference (wave propagation)|constructive interference]]). That is, synchronized firing patterns result in synchronised input into other cortical areas, which gives rise to large-amplitude oscillations of the [[local field potential]]. These large-scale oscillations can also be measured outside the scalp using [[electroencephalography]] and [[magnetoencephalography]]. The electric potentials generated by single neurons are far too small to be picked outside the scalp and EEG or MEG activity always reflects the summation of the synchronous activity of thousands or millions of neurons that have similar spatial orientation.<ref>{{cite book| author = Nunez PL, Srinivasan R | title = Electric fields of the brain: The neurophysics of EEG |publisher = Oxford University Press | year = 1981 | url = http://books.google.com/books?id=gu5qAAAAMAAJ}}</ref> Neurons in a [[neural ensemble]] rarely fire all at the exact same moment, i.e. fully synchronized. Instead, the probability of firing is rhythmically modulated such that neurons are more likely to fire at the same time, which gives rise to oscillations in their mean activity (see figure at top of page). As such, the frequency of [[macroscopic scale|large-scale]] oscillations does not need to match the firing pattern of individual neurons. Isolated cortical neurons fire regularly under certain conditions, but in the intact brain cortical cells are bombarded by highly fluctuating synaptic inputs and typically fire seemingly random. However, if the probability of a large group of neurons is rhythmically modulated at a common frequency, they will generate oscillations in the mean field (see also figure at top of page).<ref name='Wang 2010'/> Neural ensembles can generate oscillatory activity [[endogenous|endogenously]] through local interactions between excitatory and inhibitory neurons. In particular, inhibitory [[interneurons]] play an important role in producing neural ensemble synchrony by generating a narrow window for effective excitation and rhythmically modulating the firing rate of excitatory neurons.<ref>{{cite journal| author = Cardin JA, Carlen M, Meletis K, Knoblich, U, Zhang F, Deisseroth K, Tsai LH, Moore CI | title =Driving fast-spiking cells induces gamma rhythm and controls sensory responses | journal = Nature | year = 2009 | volume= 459 | issue= 7247 | pages= 663-U63 | doi=10.1038/nature08002 }}</ref> |
||
| + | |||
| + | === Macroscopic === |
||
| + | |||
| + | Neural oscillation can also arise from interactions between different brain areas. [[Propagation delay|Time delays]] play an important role here. Because all brain areas are bidirectionally coupled, these connections between brain areas form [[feedback]] loops. [[Positive feedback]] loops tends to cause oscillatory activity which frequency is inversely related to the delay time. An example of such a feedback loop is the connections between the [[thalamus]] and [[cortex]]. This thalamocortical network is able to generate oscillatory activity known as [[recurrent thalamo-cortical resonance]].<ref>{{cite journal| last = Llinas | first = Rodolfo| coauthors = | title = The neuronal basis for consciousness | journal = Phil Trans R Soc Lond | volume = 353 | pages = 1841–1849 | year = 1998}}</ref> The thalamocortical network plays an important role in the generation of [[alpha wave|alpha activity]].<ref>{{cite journal| last = Bollimunta | first = Anil| coauthors = | title = Neuronal Mechanisms and Attentional Modulation of Corticothalamic Alpha Oscillations | journal = The Journal of Neuroscience | volume = 31 |issue=13 | pages = 4935–4943 | year = 2011}}</ref><ref>{{cite journal| author = Suffczynski P, Kalitzin S, Pfurtscheller G, Lopes da Silva FH| title = Computational model of thalamo-cortical networks: dynamical control of alpha rhythms in relation to focal attention | journal = Int J Psychophysiol | volume = 43 |issue=1 | pages = 25–40 | year = 2001}}</ref> |
||
| + | |||
| + | ==Mechanisms== |
||
| + | === Neuronal properties === |
||
| + | {{see also|Action potential|Bursting}} |
||
| + | |||
| + | Scientists have identified some intrinsic [[neuron|neuronal properties]] that play an important role in generating membrane potential oscillations. In particular, [[voltage-gated ion channels]] are critical in the generation of action potentials. The dynamics of these ion channels have been captured in the well-established [[Hodgkin-Huxley model]] that describes how action potentials are initiated and propagated by means of a set of differential equations. Using [[bifurcation theory|bifurcation analysis]], different oscillatory varieties of these neuronal models can be determined, allowing for the classification of types of neuronal responses. The oscillatory dynamics of neuronal spiking as identified in the Hodgkin-Huxley model closely agree with empirical findings. In addition to periodic spiking, [[subthreshold membrane potential oscillations]], i.e. [[resonance]] behavior that does not result in action potentials, may also contribute to oscillatory activity by facilitating synchronous activity of neighboring neurons.<ref>{{cite journal | author = Llinas RR | title = The Intrinsic electrophysiological properties of mammalian neurons: A new insight into CNS function | journal = Science | volume = 242 | pages = 1654–1664 | year = 1988 | doi = 10.1126/science.3059497 | pmid = 3059497 | issue = 4886}}</ref><ref>{{cite journal | author = Llinas RR, Grace AA, Yarom Y | year = 1991 | title = In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range | journal = Proc Natl Acad Sci USA | volume = 88 | pages = 897–901 | doi = 10.1073/pnas.88.3.897 | pmid = 1992481 | issue = 3 | pmc = 50921}}</ref> Like pacemaker neurons in central pattern generators, subtypes of cortical cells fire bursts of spikes (brief clusters of spikes) rhythmically at preferred frequencies. Bursting neurons have the potential to serve as pacemakers for synchronous network oscillations, and bursts of spikes may underlie or enhance neuronal resonance.<ref name = 'Wang 2010'/> |
||
| + | |||
| + | === Network properties === |
||
| + | {{see also| Connectome|Oscillatory networks}} |
||
| + | |||
| + | Apart from intrinsic properties of neurons, [[neural network|network]] properties are also an important source of oscillatory activity. Neurons [[Neuron#Connectivity|communicate]] with one another via synapses and affect the timing of spike trains in the post-synaptic neurons. Depending on the properties of the connection, such as the coupling strength, time delay and whether coupling is [[EPSP|excitatory]] or [[IPSP|inhibitory]], the spike trains of the interacting neurons may become [[phase synchronization|synchronized]].<ref>{{cite journal | author =Zeitler M, Daffertshofer A, Gielen CCAM | year = 2009 | title =Asymmetry in pulse-coupled oscillators with delay | journal = Phys Rev E| volume = 79 | issue = 6 | article = 065203(R) | doi = 10.1103/PhysRevE.79.065203}}</ref> Neurons are locally connected, forming small clusters that are called [[neural ensemble]]s. Certain network structures promote oscillatory activity at specific frequencies. For example, neuronal activity generated by two populations of interconnected ''inhibitory'' and ''excitatory'' cells can show spontaneous oscillations that are described by the [[Wilson-Cowan model]]. |
||
| + | If a group of neurons engages in synchronized oscillatory activity, the neural ensemble can be mathematically represented as a single oscillator.<ref name = "Haken 1996"/> Different neural ensembles are coupled through long-range connections and form a network of weakly coupled oscillators at the next spatial scale. Weakly coupled oscillators can generate a range of dynamics including oscillatory activity.<ref name = "Pikovsky 2001">{{cite book | author=Pikovsky A, Rosenblum M, Kurths J | title= Synchronization: a universal concept in nonlinear sciences | year=2001 | publisher=Cambridge University Press | isbn=0-521-53352-X}}</ref> Long-range connections between different brain structures, such as the [[thalamus]] and the [[Cerebral cortex|cortex]] (see [[Recurrent thalamo-cortical resonance|thalamocortical oscillation]]), involve time-delays due to the finite [[Action potential#Propagation|conduction velocity]] of axons. Because most connections are reciprocal, they form [[feedback|feed-back loops]] that support oscillatory activity. Oscillations recorded from multiple cortical areas can become synchronized and form a large-scale network, whose dynamics and functional connectivity can be studied by means of [[frequency domain|spectral analysis]] and [[Granger causality]] measures.<ref>Andrea Brovelli, Steven L. Bressler and their colleagues, [http://www.pnas.org/cgi/reprint/101/26/9849.pdf 2004]</ref> Coherent activity of large-scale brain activity may form dynamic links between brain areas required for the integration of distributed information.<ref name = "Varela 2001"/> |
||
| − | Neural oscillations are characterized by their [[frequency]], [[amplitude]] and [[phase]]. These signal proprieties can be extracted from neural recordings using [[time-frequency analysis]]. Changes in these characteristics have been linked to various functions. In large-scale oscillations, amplitude changes are considered to result from changes in synchronization within a [[neural ensemble]], also referred to as local synchronization, and have been linked to cognitive functions such as [[perception]] and [[motor control]]. Apart from local synchronization, changes in the synchronization between oscillatory activity of distant neural ensembles has been observed, which might serve as a neural mechanism for information transfer.<ref name = "Fries 2001">{{cite journal | author = Fries P | title = A mechanism for cognitive dynamics: neuronal communication through neuronal coherence | journal = TICS | volume = 9 | pages = 474–480 | year = 2001}}</ref> |
||
| + | === Neuromodulation === |
||
| − | The study of neural oscillations belongs to the field of “neurodynamics”, an area of research in the [[cognitive science]]s that places a strong focus upon the dynamic character of neural activity in describing [[brain]] function. The term neurodynamics dates back before the 1940s,<ref>{{cite journal | author = Burrow T | title = The neurodynamics of behavior. A phylobiological foreword | journal = Philosophy of Science | volume = 10 | pages = 271–288 | year = 1943}}</ref> and is an offshoot of neuro-[[cybernetics]] using [[differential equations]] to describe neural activity patterns. Research in neurodynamics involves the interdisciplinary areas of contemporary theoretical [[neurobiology]], [[nonlinear dynamics]], [[complex adaptive systems]] and [[statistical physics]]. Neurodynamics is often contrasted with the popular computational and modular approaches of [[cognitive neuroscience]], and with the implicit or explicit [[representationalism]] in cognitive science. |
||
| + | {{main article| Neuromodulation}} |
||
| + | In addition to fast direct [[synapse|synaptic interactions]] between neurons forming a network, oscillatory activity is modulated by [[neurotransmitters]] on a much slower time scale. That is, the concentration levels of certain neurotransmitters are known to regulate the amount of oscillatory activity. For instance, [[GABA]] concentration has been shown to be positively correlated with frequency of oscillations in induced stimuli.<ref>{{cite journal | author = Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD | title = Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans | journal = Proc Nat Acad Sci USA | volume = 106 | pages = 8356–8361 | year = 2009 | doi = 10.1073/pnas.0900728106 | pmid = 19416820 | issue = 20 | pmc = 2688873}}</ref> A number of [[Nucleus (neuroanatomy)|nuclei]] in the [[brainstem]] have diffuse projections throughout the brain influencing concentration levels of neurotransmitters such as [[norepinephrine]], [[acetylcholine]] and [[serotonin]]. These neurotransmitter systems affect the physiological state, e.g., [[wakefulness]] or [[arousal]], and have a pronounced effect on amplitude of different brain waves, such as alpha activity.<ref>{{cite journal | author = Moruzzi G, Magoun HW | title = Brain stem reticular formation and activation of the EEG | journal = Electroencephalogr Clin Neurophysiol | volume = 1 | pages = 455–473 | year = 1949 | doi = 10.1016/0013-4694(49)90219-9}}</ref> |
||
| − | Neural Field Theories is a mathematical framework describing the spatio-temporal evolution of variables such as mean firing rate. In modeling the activity of large numbers of [[neurons]], the central idea is to take the density of neurons to the continuum limit, resulting in spatially continuous [[neural networks]]. Models based on these principles provide mathematical descriptions of neural oscillations and EEG rhythms and have been used to investigate visual hallucinations,<ref>Bressloff PC, Cowan JD (2003) Spontaneous pattern formation in primary visual cortex. In: J Hogan, AR Krauskopf, M di Bernado, RE Wilson (Eds.), Nonlinear dynamics and chaos: where do we go from here? </ref> and mechanisms for short-term memory and motion perception. |
||
== Mathematical description == |
== Mathematical description == |
||
| + | {{see also| Computational neuroscience}} |
||
| − | Mathematicians have identified several [[dynamical systems|dynamical]] mechanisms that generate rhythmicity. Among the most important are [[harmonic oscillator|harmonic]] (linear) oscillators, [[limit-cycle]] oscillators, and delayed-[[feedback]] oscillators. Harmonic oscillations appear very frequently in nature—examples are sound waves, the motion of a [[pendulum]], and vibrations of every sort. They generally arise when a physical system is perturbed by a small degree from a [[Principle of minimum energy|minimum-energy state]], and are well-understood mathematically. |
+ | Oscillations can often be described and analyzed using mathematics. Mathematicians have identified several [[dynamical systems|dynamical]] mechanisms that generate rhythmicity. Among the most important are [[harmonic oscillator|harmonic]] (linear) oscillators, [[limit-cycle]] oscillators, and delayed-[[feedback]] oscillators.<ref>{{cite journal | author = Buzsaki G, Draguhn A | title = Neuronal oscillations in cortical networks | journal = Science | volume = 304 | issue = 5679 | year = 2004 | pages = 1926–1929 | doi = 10.1126/science.1099745 }}</ref> Harmonic oscillations appear very frequently in nature—examples are sound waves, the motion of a [[pendulum]], and vibrations of every sort. They generally arise when a physical system is perturbed by a small degree from a [[Principle of minimum energy|minimum-energy state]], and are well-understood mathematically. Noise-driven harmonic oscillators realistically simulate alpha rhythm in the waking EEG as well as slow waves and spindles in the sleep EEG. Successful EEG analysis algorithms were based on such models. Several other EEG components are better described by limit-cycle or delayed-feedback oscillations. Limit-cycle oscillations arise from physical systems that show large deviations from [[Non-equilibrium thermodynamics|equilibrium]], whereas delayed-feedback oscillations arise when components of a system affect each other after significant time delays. Limit-cycle oscillations can be complex but there are powerful mathematical tools for analyzing them; the mathematics of delayed-feedback oscillations is primitive in comparison. Linear oscillators and limit-cycle oscillators qualitatively differ in terms of how they respond to fluctuations in input. In a linear oscillator, the frequency is more or less constant but the amplitude can vary greatly. In a limit-cycle oscillator, the amplitude tends to be more or less constant but the frequency can vary greatly. A [[cardiac cycle|heartbeat]] is an example of a limit-cycle oscillation in that the frequency of beats varies widely, while each individual beat continues to pump about the same amount of blood. |
| + | [[Computational models]] adopt a variety of abstractions in order to describe complex oscillatory dynamics observed in brain activity. Many models are used in the field, each defined at a different level of abstraction and trying to model different aspects of neural systems. They range from models of the short-term behaviour of individual neurons, through models of how the dynamics of neural circuitry arise from interactions between individual neurons, to models of how behaviour can arise from abstract neural modules that represent complete subsystems. |
||
| − | Linear oscillators and limit-cycle oscillators qualitatively differ in terms of how they respond to fluctuations in input. In a linear oscillator, the frequency is more or less constant but the amplitude can vary greatly. In a limit-cycle oscillator, the amplitude tends to be more or less constant but the frequency can vary greatly. A [[cardiac cycle|heartbeat]] is an example of a limit-cycle oscillation in that the frequency of beats varies widely, while each individual beat continues to pump about the same amount of blood. |
||
| − | == |
+ | === Single neuron model === |
| + | [[File:Simulation of hrose neuron.png|thumb|right|Simulation of a [[Hindmarsh-Rose model|Hindmarsh-Rose neuron]] showing typical [[bursting]] behavior: a fast rhythm generated by individual spikes and a slower rhythm generated by the bursts.]] |
||
| + | {{see also| Biological neuron model}} |
||
| − | Neurons generate [[action potentials]] that reflect changes in the electric membrane potential. Neurons can generate multiple action potentials in sequence forming so-called spike trains. These spike trains are the basis for [[neural coding]] and information transfer in the brain. Spike trains can form all kind of patterns, such as rhythmic spiking and [[bursting]], and are often considered oscillatory activity. |
||
| + | A model of a biological neuron is a mathematical description of the properties of nerve cells, or [[neurons]], that is designed to accurately describe and predict its biological processes. The most successful and widely-used model of neurons, the [[Hodgkin-Huxley model]], is based on data from the [[squid giant axon]]. It is a set of nonlinear ordinary differential equations that approximates the electrical characteristics of a neuron, in particular the generation and propagation of [[action potentials]]. The model is very accurate and detailed and [[Alan Lloyd Hodgkin|Hodgkin]] and [[Andrew Huxley|Huxley]] received the 1963 Nobel Prize in physiology or medicine for this work. |
||
| − | === Mechanisms === |
||
| − | Scientists have identified some intrinsic [[neuron|neuronal properties]] that can result in membrane potential oscillations. In particular, [[voltage-gated ion channels]] are critical in the generation of action potentials. The dynamics of these ion channels have been captured in the well-established [[Hodgkin-Huxley model]] that describes how action potentials are initiated and propagated by means of a set of differential equations. Using [[bifurcation theory|bifurcation analysis]], different oscillatory regimes of these neuronal models can be determined, allowing for the classification of types of neuronal responses. The oscillatory dynamics of neuronal spiking as identified using mathematical models closely agree with empirical findings. In addition to periodic spiking, [[subthreshold membrane potential oscillations]], i.e. fluctuations that do not result in action potentials, may also contribute to oscillatory activity by facilitating synchronous activity of neighboring neurons.<ref>{{ cite journal | author = Llinas RR | title = The Intrinsic electrophysiological properties of mammalian neurons: A new insight into CNS function | journal = Science | volume = 242 | pages = 1654–1664 | year = 1988 | doi = 10.1126/science.3059497 | pmid = 3059497 | issue = 4886}}</ref><ref>{{ cite journal | author = Llinas RR, Grace AA, Yarom Y | year = 1991 | title = In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range | journal = Proc Natl Acad Sci USA | volume = 88 | pages = 897–901 | doi = 10.1073/pnas.88.3.897 | pmid = 1992481 | issue = 3 | pmc = 50921}}</ref> |
||
| + | The mathematics of the Hodgkin-Huxley model are quite complicated and several simplifications have been proposed, such as the [[FitzHugh-Nagumo model]] and the [[Hindmarsh-Rose model]]. Such models only capture the basic neuronal dynamics, such as rhythmic spiking and [[bursting]], but are more computationally efficient. This allows the simulation of a large number of interconnected neurons that form a [[neural network]]. |
||
| − | === Activity pattern === |
||
| − | Neuronal spiking can be classified by their activity patterns. The excitability of neurons can be subdivided in Class 1 and 2. Class 1 neurons can generate action potentials with arbitrarily low frequency depending on the input strength, whereas Class 2 neurons generate action potentials in a certain frequency band, which is relative insensitive to changes in input strength<ref>{{cite book | author = Izhikevich EM | title = Dynamical systems in neuroscience | publisher = The MIT Press | city = Cambridge, Massachusetts | year = 2007}}</ref>. |
||
| − | === |
+ | === Spiking model === |
| + | {{see also| Neural network}} |
||
| − | Neuronal spiking is the basis for information transfer in the brain. Different types of coding schemes have been proposed, such as [[rate coding]] and [[temporal coding]]. |
||
| + | A neural network model describes a population of physically interconnected neurons or a group of disparate neurons whose inputs or signalling targets define a recognizable circuit. These models aim to describe how the dynamics of neural circuitry arise from interactions between individual neurons. Local interactions between neurons can result in the synchronization of spiking activity and form the basis of oscillatory activity. In particular, models of interacting [[pyramidal cells]] and inhibitory [[interneurons]] have been shown to generate brain rhythms such as [[gamma wave|gamma activity]].<ref>{{cite journal |author=Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH |title=Inhibition-based rhythms: experimental and mathematical observations on network dynamics |journal= Int J Psychophysiol |volume=38 |pages=315–336 |year=2000}}</ref> |
||
| − | == Large-scale oscillations == |
||
| + | === Neural mass model === |
||
| − | Apart for single neurons that can generate oscillatory spike trains, groups of neurons often reveal oscillatory behavior. These large-scale oscillations arise to synchronized activity of multiple neurons. Experimentally these oscillations can be measured by fluctuation in the [[local field potential]] or by mean of [[EEG]] and [[MEG]]. |
||
| + | [[File:NeuralMassSimulation.png|thumb|right|Simulation of a neural mass model showing network spiking during the onset of a [[Epileptic seizure|seizure]].<ref>{{cite journal |author=Wendling F, Bellanger JJ, Bartolomei F, Chauvel P |title=Relevance of nonlinear lumped-parameter models in the analysis of depth-EEG epileptic signals |journal= Biol Cybern |volume=83 |pages=367–378 |year=2000}}</ref> As the gain A is increased the network starts to oscillate at 3Hz.]] |
||
| + | {{see also| Wilson-Cowan model}} |
||
| − | === Mechanisms === |
||
| − | ==== Network properties ==== |
||
| − | Apart from intrinsic properties of neurons, [[neural network|network]] properties are also an important source of oscillatory activity. Neurons are locally connected, forming small clusters that are called [[neural ensemble|neural ensembles]]. Certain network structures promote oscillatory activity at specific frequencies. This is determined by the type of neurons, i.e. ''excitatory'' or ''inhibitory'' [[neurons]], time delays and the coupling function. [[central pattern generator|Central pattern generators]] are a well-known example of neural networks that can endogenously produce rhythmic patterned outputs. Neural synchronization is the process by which the activity of two or more [[neuron]]s or [[neural ensemble]]s tend to [[neural oscillations|oscillate]] with a repeating sequence of relative [[phase (waves)|phase]] angles. Mathematically, neural ensembles can be considered [[Kuramoto model|weakly coupled oscillators]], a type of system that readily allows for synchronized oscillatory activity.<ref>{{cite book | author=Pikovsky A, Rosenblum M, Kurths J | title= Synchronization: a universal concept in nonlinear sciences | year=2001 | publisher=Cambridge University Press}}</ref><ref>{{cite book | author=Haken H | title= Principles of brain functioning | year=1996 | publisher=Springer}}</ref> |
||
| + | Neural field models are another important tool in studying neural oscillations and are a mathematical framework describing evolution of variables such as mean firing rate in space and time. In modeling the activity of large numbers of [[neurons]], the central idea is to take the density of neurons to the continuum limit, resulting in spatially continuous [[neural networks]]. Instead of modelling individual neurons, this approach approximates a group of neurons by its average properties and interactions. It is based on the [[mean field theory|mean field approach]], an area of [[statistical physics]] that deals with large-scale systems. Models based on these principles have been used to provide mathematical descriptions of neural oscillations and EEG rhythms. They have for instance been used to investigate visual hallucinations.<ref>Bressloff PC, Cowan JD (2003) Spontaneous pattern formation in primary visual cortex. In: J Hogan, AR Krauskopf, M di Bernado, RE Wilson (Eds.), Nonlinear dynamics and chaos: where do we go from here?</ref> |
||
| − | Synchronized activity of a large number of neurons results in electromagnetic fields that can be measured outside the scalp with [[electroencephalography]] and [[magnetoencephalography]]. Using these techniques, synchronized neural activity have been observed throughout the [[central nervous system]] and during various tasks. Neural synchronization can be modulated by task constraints, such as [[attention]], and is thought to play a role in [[neural binding|feature binding]],<ref>{{cite journal | author = Singer W | title = Synchronization of cortical activity and its putative role in information processing and learning | journal = Annu Rev Physiol | volume = 55 | pages = 349–374 | year = 1993}}</ref> neuronal communication,<ref name = "Fries 2001"/> and [[motor coordination]].<ref>{{cite journal | author = Schnitlzer A, Gross J | title = Normal and pathological oscillatory communication in the brain | journal = Nat Rev Neurosci | volume = 6 | pages = 285–296 | year = 2005}}</ref> |
||
| − | + | === Kuramoto model === |
|
| + | [[File:KuramotoModel.ogv|thumb|240px| right|Simulation of [[Kuramoto model]] showing neural synchronization and oscillations in the mean field]] |
||
| − | Connections between different brain structures, for instance the [[thalamus]] and the [[cortex]], can form loops that support oscillatory activity. Oscillations recorded from multiple cortical areas can become synchronized and form a large-scale network, whose dynamics and functional connectivity can be studied by means of [[frequency domain|spectral analysis]] and [[Granger causality]] measures.<ref>Andrea Brovelli, Steven L. Bressler and their colleagues, [http://www.pnas.org/cgi/reprint/101/26/9849.pdf 2004]</ref> Coherent activity of large-scale brain activity might form dynamic links between brain areas required for the integration of distributed information.<ref>{{cite journal | author = Varela F, Lachaux JP, Rodriguez E, Martinerie J | title = The brainweb: phase synchronization and large-scale integration | journal = Nat Rev Neurosci | volume = 2 | pages = 229–239 | year = 2001 | doi = 10.1038/35067550 | pmid = 11283746 | issue = 4}}</ref> |
||
| + | {{main|Kuramoto model}} |
||
| − | ==== Neurotransmitters ==== |
||
| − | Certain [[neurotransmitters]] are known to regulate the amount of oscillatory activity. [[GABA]] concentration has been shown to be positively correlated with frequency of oscillations in induced stimuli. The exact relationship, however, can only be resolved with further pharmacological research on how GABA concentrations affect oscillatory dynamics of single neurons and [[local field potentials]] of ensembles of neurons.<ref>{{ cite journal | author = Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD | title = Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans | journal = Proc Nat Acad Sci USA | volume = 106 | pages = 8356–8361 | year = 2009 | doi = 10.1073/pnas.0900728106 | pmid = 19416820 | issue = 20 | pmc = 2688873}}</ref> |
||
| + | The [[Kuramoto model]] of coupled phase oscillators<ref>{{cite book | author=Kuramoto Y | title= Chemical Oscillations, Waves, and Turbulence | year=1984 | publisher=Dover Publications }}</ref> is one of the most abstract and fundamental model used to investigate neural oscillations and sychronization. It captures the activity of a local system (e.g., a single neuron or neural ensemble) by its circular [[phase (waves)|phase]] alone and hence ignores the amplitude of oscillations (amplitude is constant).<ref>{{cite journal | author = Ermentrout B | title = An introduction to neural oscillators | journal = In F Ventriglia (ed.), Neural Modeling and Neural Networks | year = 1994 | pages = 79–110 }}</ref> Interactions amongst these oscillators are introduced by a simple algebraic form (such as a [[trigonometric functions|sin]] function) and collectively generate a dynamical pattern at the global scale. The Kuramoto model is widely used to study oscillatory brain activity and several extensions have been proposed that increase its neurobiological plausibility, for instance by incorporating topological properties of local cortical connectivity.<ref>{{cite journal | doi = 10.3389/fnhum.2010.00190 | author = Breakspear M, Heitmann S, Daffertshofer A | title = Generative models of cortical oscillations: Neurobiological implications of the Kuramoto model | journal = Front Hum Neurosc | volume = 4 | article = 190 | year = 2010 }}</ref> In particular, it describes how the activity of a group of interactioning neurons can become synchronized and generate large-scale oscillations. Simulations using the Kuramoto model with realistic long-range cortical connectivity and time-delayed interactions reveal the emergence of slow patterned fluctuations that reproduce resting-state [[Blood-oxygen-level dependent|BOLD]] functional maps, which can be measured using [[fMRI]].<ref>{{cite journal | doi = 10.1016/j.neuroimage.2011.04.010 | author = Cabral J, Hugues E, Sporns O, Deco G | title = Role of local network oscillations in resting-state functional connectivity | journal = Neuroimage | year = 2011 | pmid=21511044}}</ref> |
||
| − | ===Activity patterns=== |
||
| + | {{-}} |
||
| − | ====Spontaneous activity==== |
||
| − | Spontaneous activity is [[brain]] activity in the absence of an explicit task, such as sensory input or motor output. It is opposed to induced activity, i.e. brain activity that is induced by sensory stimuli or motor responses. The term ''ongoing brain activity'' is used in [[electroencephalography]] and [[magnetoencephalography]] for those signal components that are not associated with the processing of a [[stimulus (physiology)|stimulus]] or the occurrence of specific other events, such as moving a body part, i.e. that do not form [[evoked potential]]s/[[evoked field]]s, [[event-related potential]]s, or induced activity. Spontaneous activity is usually considered to be [[Signal noise|noise]] if one is interested in stimulus processing, but might be informative regarding the current mental state of the person (e.g. wakefulness, alertness) and is often used in [[sleep]] research. Certain types of oscillatory activity, such as [[alpha wave]]s, are part of spontaneous activity. |
||
| + | == Activity patterns == |
||
| − | Most neuroscience studies have focused on the brain’s response to a task or stimulus. However, the brain is very active even in the absence of explicit input or output. Spontaneous activity is investigated using a paradigm that requires subjects to open and close |
||
| + | |||
| − | their eyes at fixed intervals while [[fMRI]] or [[EEG]] activity is recorded. In case of fMRI, spontaneous fluctuations in the blood oxygen level dependent ([[BOLD]]) signal reveal correlation patterns that are linked to different resting states.<ref>{{citation |title=Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging |author=Fox MD, Raichle ME |year=2007 |journal=Nat Neurosci Rev |volume=8 |pages=700–711 }}</ref> In EEG research, spontaneous fluctuations of [[neural oscillations|oscillatory activity]] are investigated and power changed in different EEG bands show correlations with the distributed patterns of fMRI activity.<ref>{{citation |title=Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging |author=Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A|year=2003 |journal=PNAS |volume=100 |pages=11053–11058}}</ref> Research on spontaneous activity led to the hypothesis that specific brain regions constitute a network supporting a default mode of brain functioning.<ref>{{citation |title=Functional connectivity in the resting brain: A network analysis of the default mode hypothesis |author=Greicius MD, Krasnow B, Reiss AL, Menon V |year=2003 |journal=PNAS |volume=100 |pages=253–258}}</ref> |
||
| + | Both single and groups of neurons can generate oscillatory activity spontaneously. In addition, they may show oscillatory responses to perceptual input or motor output. Some types of neurons will fire rhythmically in the absence of any synaptic input. Likewise, brain wide activity reveals oscillatory activity while subjects do not engage in any activity, so-called resting-state activity. These ongoing rhythms can change in different ways in response to perceptual input or motor output. Oscillatory activity may respond by increases or decreases in frequency and amplitude or show a temporary interruption, which is referred to as phase resetting. In addition, external activity may not interact with ongoing activity at all, resulting in an additive response. |
||
| + | {{Gallery |
||
| − | ====Evoked activity==== |
||
| + | |title=Oscillatory responses |
||
| − | The term evoked activity is used in [[electroencephalography]] and [[magnetoencephalography]] for certain types of [[stimulus (physiology)|stimulus]]-related activity. The following explanation is for electroencephalographic activity (EEG), but the concept is the same in magnetoencephalography (MEG). |
||
| + | |width=210 |
||
| + | |height=170 |
||
| + | |lines=2 |
||
| + | |align=center |
||
| + | |File:Freq response.png|The [[frequency]] of ongoing oscillatory activity is increased between t1 and t2. |
||
| + | |File:Amp response.png|The [[amplitude]] of ongoing oscillatory activity is increased between t1 and t2. |
||
| + | |File:Phase resetting.png|The [[phase (waves)|phase]] of ongoing oscillatory activity is reset at t1. |
||
| + | |File:Additive response.png|Activity is linearly added to ongoing oscillatory activity between t1 and t2. |
||
| + | }} |
||
| + | === Ongoing activity === |
||
| − | [[Evoked potential]]s and [[event-related potential]]s are obtained from the electroencephalogram by stimulus-locked averaging. As a consequence, those signal components that are the same in each single measurement are conserved and all others are averaged out. That is, event-related potentials only reflect oscillations in brain activity that are [[phase (waves)|phase]]-locked to the stimulus or event. Evoked activity is often considered to be independent from ongoing brain activity although this is an ongoing debate.<ref>{{cite journal |title=Dynamic brain sources of visual evoked responses|author=Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ |year=2002 |journal=Science |volume=295 |pages=690–694}}</ref> |
||
| + | Spontaneous activity is [[brain]] activity in the absence of an explicit task, such as sensory input or motor output, and hence also referred to as [[resting-state activity]]. It is opposed to induced activity, i.e. brain activity that is induced by sensory stimuli or motor responses. The term ''ongoing brain activity'' is used in [[electroencephalography]] and [[magnetoencephalography]] for those signal components that are not associated with the processing of a [[stimulus (physiology)|stimulus]] or the occurrence of specific other events, such as moving a body part, i.e. events that do not form [[evoked potential]]s/[[evoked field]]s, or induced activity. Spontaneous activity is usually considered to be [[Signal noise|noise]] if one is interested in stimulus processing. However, spontaneous activity is considered to play a crucial role during brain development, such as in network formation and synaptogenesis. Spontaneous activity may be informative regarding the current mental state of the person (e.g. wakefulness, alertness) and is often used in sleep research. Certain types of oscillatory activity, such as [[alpha wave]]s, are part of spontaneous activity. Statistical analysis of power fluctuations of alpha activity reveals a bimodal distribution, i.e. a high- and low-amplitude mode, and hence shows that resting-state activity does not just reflect a [[Gaussian noise|noise]] process.<ref>{{Cite journal |title=Bistability and non-Gaussian fluctuations in spontaneous cortical activity |author=Freyer F, Aquino K, Robinson PA, Ritter P, Breakspear M | year=2009 |journal=J Neurosci |volume=29 |issue=26 |pages=8512–8524 |doi=10.1523/JNEUROSCI.0754-09.2009}}</ref> In case of fMRI, spontaneous fluctuations in the [[Blood-oxygen-level dependent]] (BOLD) signal reveal correlation patterns that are linked to resting states networks, such as the [[default network]].<ref>{{Cite journal |doi=10.1038/nrn2201 |title=Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging |author=Fox MD, Raichle ME |year=2007 |journal=Nat Neurosci Rev |volume=8 |pages=700–711 |issue=9}}</ref> The temporal evolution of resting state networks is correlated with fluctuations of oscillatory EEG activity in different frequency bands.<ref>{{Cite journal |title=Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging |author=Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A|year=2003 |journal=PNAS |volume=100 |issue=19 |pages=11053–11058 |pmid=12958209 |pmc=196925 |doi=10.1073/pnas.1831638100}}</ref> |
||
| − | ====Induced activity==== |
||
| − | Next to evoked activity, neural activity related to stimulus processing may result in induced activity. Induced activity refers to changes in ongoing brain activity induced by processing of stimuli or movement preparation. A well-studied type of induced activity is amplitude change in oscillatory activity. For instance, [[Gamma wave|gamma activity]] often increases during increased mental activity such as during object representation.<ref>{{cite journal|title=Oscillatory gamma activity in humans and its role in object representation|author=Tallon-Baudry C, Bertrand O |year=1999 |journal=Trends Cogn Sci |volume=3 |pages=151–162}}</ref> Because induced responses may have different phases across measurements and therefore would cancel out during averaging, they can only be obtained using [[time-frequency analysis]]. Induced activity generally reflects the activity of numerous neurons and amplitude changes in oscillatory activity are thought to arise from the synchronization of neural activity, e.g. synchronization of spikes or membrane potential fluctuations of individual neurons. Increases and decreases in oscillatory activity are therefore often referred to as event-related synchronization and desynchronization.<ref name = "Pfurtscheller 1999">{{cite journal |title=Event-related EEG/MEG synchronization and desynchronization: basic principles |author=Pfurtscheller G, da Silva FHL |year=1999 |journal=Clin Neurophysiol |volume=110 |pages=1842–1857}}</ref> |
||
| + | Ongoing brain activity may also have an important role in perception, as it may interact with activity related to incoming stimuli. Indeed, [[EEG]] studies suggest that visual perception is dependent on both the phase and amplitude of cortical oscillations. For instance, the amplitude and phase of alpha activity at the moment of visual stimulation predicts whether a weak stimulus will be perceived by the subject.<ref>{{cite journal | author = Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T | journal = J Neurosci | volume = 29 | doi = 10.1523/JNEUROSCI.3963-08.2009 | title = To see or not to see: Prestimulus α phase predicts visual awareness | year = 2009 | pages = 2725–32 | pmid = 19261866 | issue = 9}}</ref><ref>{{cite journal | author = Busch NA, Dubois J, VanRullen R | journal = J Neurosci | volume = 29 | doi = 10.1523/jneurosci.0113-09.2009 | title = The phase of ongoing EEG oscillations predicts visual perception | year = 2009 | pages = 7869–76 | pmid = 19535598 | issue = 24}}</ref><ref>{{cite journal | author = van Dijk H, Schoffelen JM, Oostenveld R, Jensen O | journal = J Neurosci | volume = 28 | doi = 10.1523/jneurosci.1853-07.2008 | title = Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability | year = 2008 | pages = 1816–1823 | issue = 8}}</ref> |
||
| − | === Function === |
||
| − | ==== Visual system ==== |
||
| − | Neuronal oscillations became a hot topic in [[neuroscience]] in the 1990s when the studies of the visual system of the brain by Gray, Singer and others appeared to support the [[neural binding]] hypothesis.<ref>{{cite journal | author = Singer W, Gray CM | title = Visual feature integration and the temporal correlation hypthesis | journal = Ann Rev Neurosci | volume = 18 | pages = 555–586 | year = 1995 | doi = 10.1007/BF01797193 | pmid = 7605074}}</ref> According to this idea, [[neural synchronization|synchronous oscillations]] in neuronal ensembles bind neurons representing different features of an object. For example, when a person looks at a tree, visual cortex neurons representing the tree trunk and those representing the branches of the same tree would oscillate in synchrony to form a single representation of the tree. Some scientists have questioned whether these oscillations are prominent, or relevant, in ensembles that consider only action potential activity.<ref>Neuroreport. 1992 Apr;3(4):369–72</ref> These oscillations are, however, prominent in differential [[local field potential|LFP]] recordings taken between upper and lower cortical layers, which suggests a local current, but not action potential, basis for their origin.<ref>J Clin Neurophysiol. 2000 Jul;17(4):341–60</ref> |
||
| + | === Frequency response === |
||
| − | [[EEG]] studies suggest that visual perception is phase dependent as well as amplitude dependent. In a study in which human subjects were stimulated with flashes of light, it was found that phase dependence accounted for 16% of variability in the measured response to stimuli. The results suggest that ongoing oscillations provide a temporal reference for visual perception via precise spike timing.<ref>{{cite journal | author = Busch NA, Dubois J, VanRullen R | journal = J Neurosci | volume = 29 | doi = 10.1523/jneurosci.0113-09.2009 | title = The Phase of Ongoing EEG Oscillations Predicts Visual Perception | year = 2009 | pages = 7869 | pmid = 19535598 | issue = 24 | unused_data = |pages: 7869-7876|year: 2009}}</ref> EEG evidence also suggests that local oscillatory bursts display significant patterns of synchrony. During flickering light simulation of human subjects, quantifiable oscillatory patterns of synchronicity had significantly higher degrees of co-occurrence during stimulation than the background levels of synchronicity. This suggests that during visual stimulation, oscillatory patterns are reorganized in the [[visual cortex]] and propagate throughout the brain.<ref>{{cite journal | author = Vialatte FB, Dauwels J, Maurice M, Yamaguchi Y, Cichocki A | title = On the synchrony of steady state visual evoked potentials and oscillatory burst events | journal = Cogn Neurodynamics | volume = 3 | pages = 251–261 | year = 1009 | doi = 10.1007/s11571-009-9082-4}}</ref> |
||
| + | In response to input, a neuron or neuronal ensemble may change the frequency at which it oscillates. This is very common in single neurons where the firing rate depends on the summed activity it receives. This is referred to as [[rate coding]]. Frequency changes are also commonly observed in central pattern generators and directly relate to the speed of motor activities, such as step frequency in walking. Changes in frequency are not so common in oscillatory activity involving different brain areas, as the frequency of oscillatory activity is often related to the time delays between brain areas. |
||
| − | Evidence suggests that the visual system of children is less entrained by incoming information resulting in less synchronized neural responses. Adults primarily rely on sparse representations formed through experience based temporally synchronized neural interactions. In older age, declines in neuronal density and neurotransmitter chemicals increase the reliance on temporally synchronizing processing.<ref>{{ cite journal | author = Werkle-Bergner M, Shing YL, Muller V, Li SC, Lindenberger U | title = EEG gamma-band synchronization in visual coding from childhood to old age: Evidence from evoked power and inter-trial phase locking | journal = Clin Neurophysiol | volume = 120 | pages = 1291–1302 | year = 2009 | doi = 10.1016/j.clinph.2009.04.012 | pmid = 19482545 | issue = 7}}</ref> |
||
| − | + | === Amplitude response === |
|
| − | Neural oscillations may have different functional roles in different brain areas, and their functional role continues to be a matter of debate. Neural oscillations have been hypothesized to be involved in the [[sense of time]]<ref>{{ cite journal | author = Buhusi CV, Meck WH | year = 2005 | title = What makes us tick? Functional and neural mechanisms of interval timing | journal = Nat Rev Neurosci | volume = 6 | pages = 755–65 | doi = 10.1038/nrn1764 | pmid = 16163383 | issue = 10}}</ref> and in somatosensory perception<ref>{{cite journal | author = Ahissar E, Zacksenhouse M | year = 2001 | title = Temporal and spatial coding in the rat vibrissal system | journal = Prog Brain Res | volume = 130 | pages = 75–87 | doi = 10.1016/S0079-6123(01)30007-9 | pmid = 11480290}}</ref> among other functions. |
||
| + | Next to evoked activity, neural activity related to stimulus processing may result in induced activity. Induced activity refers to modulation in ongoing brain activity induced by processing of stimuli or movement preparation. Hence, they reflect an indirect response in contrast to evoked responses. A well-studied type of induced activity is amplitude change in oscillatory activity. For instance, [[Gamma wave|gamma activity]] often increases during increased mental activity such as during object representation.<ref>{{cite journal|doi=10.1016/S1364-6613(99)01299-1|title=Oscillatory gamma activity in humans and its role in object representation|author=Tallon-Baudry C, Bertrand O |year=1999 |journal=Trends Cogn Sci |volume=3 |pages=151–162}}</ref> Because induced responses may have different phases across measurements and therefore would cancel out during averaging, they can only be obtained using [[time-frequency analysis]]. Induced activity generally reflects the activity of numerous neurons: amplitude changes in oscillatory activity are thought to arise from the synchronization of neural activity, for instance by synchronization of spike timing or membrane potential fluctuations of individual neurons. Increases in oscillatory activity are therefore often referred to as event-related synchronization, while decreases are referred to as event-related desynchronization <ref name = "Pfurtscheller 1999">{{cite journal |doi=10.1016/S1388-2457(99)00141-8 |title=Event-related EEG/MEG synchronization and desynchronization: basic principles |author=Pfurtscheller G, da Silva FHL |year=1999 |journal=Clin Neurophysiol |volume=110 |pages=1842–1857 |pmid=10576479}}</ref> |
||
| − | Gilles Laurent and colleagues that showed [[neural synchronization|oscillatory synchronization]] has an important functional role in odor perception and identified some mechanisms by which this function is established. That is, different odors lead to different subsets of neurons firing on different sets of oscillatory cycles<ref>{{cite journal | author = Wehr M, Laurent G | title = Odour encoding by temporal sequences of firing in oscillating neural assemblies | journal = Nature | volume = 384 | pages = 162–166 | year = 1996 | doi = 10.1038/384162a0 | pmid = 8906790 | issue = 6605 }}</ref> and the oscillations can be disrupted by [[GABA]] blocker [[picrotoxin]].<ref>{{cite journal | author = MacLeod K, Laurent G | title = Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies | journal = Science | volume = 274 | pages = 976–979 | year = 1996 | doi = 10.1126/science.274.5289.976 | pmid = 8875938 | issue = 5289 }}</ref> Disruption of the oscillatory synchronization leads to impairment of behavioral discrimination of chemically similar odorants in bees<ref>{{cite journal | author = Stopfer M, Bhagavan S, Smith BH, Laurent G | title = Impaired odour discrimination on desynchronization of odour-encoding neural assemblies | journal = Nature | volume = 390 | pages = 70–74 | year = 1997 | doi = 10.1038/36335 | pmid = 9363891 | issue = 6655 }}</ref> and to more similar responses across odors in downstream β-lobe neurons.<ref>{{cite journal | author = MacLeod K, Bäcker A, Laurent G | title = Who reads temporal information contained across synchronized and oscillatory spike trains? | journal = Nature | volume = 395 | pages = 693–698 | year = 1998 | doi = 10.1038/27201 | pmid = 9790189 | issue = 6703 }}</ref> |
||
| − | + | === Phase resetting === |
|
| − | Oscillations have been commonly reported in the motor system. Pfurtscheller and colleagues found a reduction in [[alpha]] (8–12 Hz) and [[Beta wave|beta]] (13–30 Hz) oscillations in [[EEG]] activity when subjects made a movement.<ref>{{cite journal | author = Pfurtscheller G, Aranibar A | title = Event-related cortical desynchronization detected by power measurements of scalp EEG | journal = Electroencephalogr Clin Neurophysiol | volume = 42 | pages = 817–826 | year = 1977 | pmid = 67933 | issue = 6}}</ref><ref name = "Pfurtscheller 1999"/> Using intra-cortical recordings, Murthy and Fetz found similar oscillations in monkey cortex when the monkeys performed motor acts that required significant attention (retrieval of raisins from unseen locations).<ref>{{cite journal | author = Murthy VN, Fetz EE | title = Oscillatory activity in sensorimotor cortex of awake monkeys: Synchronization of local field potentials and relation to behavior | journal = J Neurophysiol | volume = 76 | pages = 3949–3967 | year = 1996 | pmid = 8985892 | issue = 6}}</ref> Similar findings were reported by the groups of John Donoghue and Roger Lemon.<ref>{{cite journal | author = Baker SN, Olivier E, Lemon RN | title = Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation | journal = J Physiol | volume = 501 | pages = 225–241 | year = 1997 | doi = 10.1111/j.1469-7793.1997.225bo.x | pmid = 9175005 | pmc = 1159515}}</ref><ref>{{cite journal | author = Sanes JN, Donoghue JP| title = Oscillations in local-field potentials of the primate motor cortex during voluntary movement | journal = PNAS | volume = 90 | pages = 4470–4474 | year = 1993 | doi = 10.1073/pnas.90.10.4470 | pmid = 8506287 | issue = 10 | pmc = 46533}}</ref> Recently it was found that these oscillations propagate as waves across the surface of the motor cortex along dominant spatial axes characteristic of the local circuitry of the motor cortex.<ref>{{cite journal | author = Rubino, D; Robbins, KA; Hatsopoulos, NG| title = Propagating waves mediate information transfer in the motor cortex | journal = Nat Neurosci | volume = 9 | pages = 1549–1557 | year = 2006 | doi = 10.1038/nn1802 | pmid = 17115042 | issue = 12}}</ref> |
||
| + | Another possibility is that input to a neuron or neuronal ensemble resets the phase of ongoing oscillations.<ref>{{cite book | author = Tass PA | title = Phase resetting in medicine and biology: stochastic modelling and data analysis | year = 2007 | publisher = Springer-Verlag | location = Berlin Heidelberg | ISBN = 3-540-65697-9}}</ref> Phase resetting is very common in single neurons where spike timing is adjusted to neuronal input. For instance, a neuron may start to spike at a fixed delay in response to periodic input, which is referred to as phase locking.<ref name = "Izhikevich 2007"/> Phase resetting may also occur at the level of neuronal ensembles when the phases of multiple neurons are adjusted simultaneously. Phase resetting of ongoing ensemble oscillations gives an alternative explanation for [[event-related potentials]] obtained by averaging multiple EEG trials with respect to the onset of a stimulus or event.<ref>{{cite journal | author = Mäkinen V, Tiitinen H, May P | title = Auditory event-related responses are generated independently of ongoing brain activity | journal = NeuroImage | volume = 24 | pages = 961–968 | year = 2005}}</ref> That is, if the phase of ongoing oscillations is reset to a fixed phase over multiple trials, oscillations will no longer average out but add up to give rise to an event-related potential. Moreover, phase resetting or phase locking is also fundamental for the synchronization of different neurons or different brain regions.<ref name = "Pikovsky 2001"/><ref name = "Varela 2001"/> In this case the timing of spikes becomes phase locked to the activity of other neurons instead of to external input. |
||
| − | Oscillatory rhythms at 10 Hz have been recorded in inferior olive and might be central in motor timing.<ref name = "Llinas 1986"/> These oscillations are also observed in motor output of physiological [[tremor]]<ref>{{cite journal | author = Allum JHJ, Dietz V, Freund HJ| title = Neuronal mechanisms underlying physiological tremor | journal = J Neurophysiol | volume = 41 | pages = 557–571 | year = 1978 | pmid = 660226 | issue = 3}}</ref> and when performing slow finger movements.<ref>{{cite journal | author = Vallbo AB, Wessberg J| title = Organization of motor output of slow finger movements in man| journal = J Physiol | volume = 469 | pages = 673–691 | year = 1993 | pmid = 8271223 | pmc = 1143894}}</ref> These findings might indicate that the human brain controls continuous movements intermittently. In support, it was shown that 6- to 9-Hz pulsatile velocity changes of slow finger movements are directly correlated to oscillatory activity in a cerebello-thalamo-cortical loop that might represent a neural mechanism for the intermittent motor control.<ref>{{cite journal | author = Gross J, Timmermann J, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A | title = The neural basis of intermittent motor control in humans| journal = PNAS | volume = 99 | pages = 2299–2302 | year = 2002 | doi = 10.1073/pnas.032682099 | pmid = 11854526 | issue = 4 | pmc = 122359}}</ref> |
||
| − | + | === Additive response === |
|
| + | {{see also| evoked potential}} |
||
| − | Neural oscillations are extensively linked to memory function, in particular [[theta]] activity. Theta rhythms are very strong in rodent hippocampi and entorhinal cortex during learning and memory retrieval, and are believed to be vital to the induction of [[long-term potentiation]], a potential cellular mechanism of learning and memory. The coupling between theta and [[gamma]] activity is thought to be vital for memory functions.<ref>{{ cite book | author = Buszaki G | title = Rhythms of the brain | year = 2006 | publisher = Oxford University Press}}</ref> The tight coordination of spike timing of single neurons with the local theta oscillations is linked to successful memory formation in humans, as more stereotyped spiking predicts better memory.<ref>{{ cite journal | author = Rutishauser U, Ross IB, Mamelak AN, Schuman EM | title = Human memory strength is predicted by theta-frequency phase-locking of single neurons | year = 2010 | journal = Nature | volume=464 | pages = 903-907}}</ref> |
||
| + | The term evoked activity is used in [[electroencephalography]] and [[magnetoencephalography]] for responses in brain activity that are directly related to [[stimulus (physiology)|stimulus]]-related activity. [[Evoked potential]]s and [[event-related potential]]s are obtained from the electroencephalogram by stimulus-locked averaging, i.e. averaging different trials at fixed latencies around the presentation of a stimulus. As a consequence, those signal components that are the same in each single measurement are conserved and all others, i.e. ongoing or spontaneous activity, are averaged out. That is, event-related potentials only reflect oscillations in brain activity that are [[phase (waves)|phase]]-locked to the stimulus or event. Evoked activity is often considered to be independent from ongoing brain activity although this is an ongoing debate.<ref>{{cite journal |doi=10.1126/science.1066168 |title=Dynamic brain sources of visual evoked responses|author=Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ |year=2002 |journal=Science |volume=295 |pages=690–694 |pmid=11809976}}</ref> |
||
| − | === Relevance === |
||
| − | ==== Brain-computer interface ==== |
||
| − | Pesaran and colleagues<ref>{{ cite journal | author = Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA | year = 2002 | title = Temporal structure in neuronal activity during working memory in macaque parietal cortex | journal = Nat Neurosci | volume = 5 | pages = 805–811 | doi = 10.1038/nn890 | pmid = 12134152 | issue = 8}}</ref> suggested that neural oscillations can be used as a control signal for [[brain-computer interface]]s because oscillatory pattern depends on the direction of movement that the monkey prepares to execute. A recent study of Rickert and colleagues<ref>{{ cite journal | author = Rickert J, Oliveira SC, Vaadia E, Aertsen A, Rotter S, Mehring C | year = 2005 | journal = J Neurosci | volume = 25 | pages = 8815–8824 | doi = 10.1523/JNEUROSCI.0816-05.2005 | pmid = 16192371 | title = Encoding of movement direction in different frequency ranges of motor cortical local field potentials. | issue = 39}}</ref> supports this suggestion. |
||
| + | ==Function== |
||
| − | ==== Pathological oscillations ==== |
||
| + | |||
| − | Specific types of neural oscillations may also appear in pathological situations, such as [[Parkinson's disease]] or [[epilepsy]]. Interestingly, these pathological oscillations often consist of a "perverted" version of a normal oscillation. For example, one of the best known type is the [[Spike and wave|Spike and Wave]] oscillation, which is typical of generalized or absence epileptic seizures, and which mechanisms are very close to that of the sleep spindle oscillations. |
||
| + | Neural synchronization can be modulated by task constraints, such as [[attention]], and is thought to play a role in [[neural binding|feature binding]],<ref name = "Singer 1993">{{cite journal | doi = 10.1146/annurev.ph.55.030193.002025 | author = Singer W | title = Synchronization of cortical activity and its putative role in information processing and learning | journal = Annu Rev Physiol | volume = 55 | pages = 349–374 | year = 1993 | pmid = 8466179}}</ref> neuronal communication,<ref name = "Fries 2001"/> and [[motor coordination]].<ref name = "Schnitzler 2005"/> Neuronal oscillations became a hot topic in [[neuroscience]] in the 1990s when the studies of the visual system of the brain by Gray, Singer and others appeared to support the [[neural binding]] hypothesis.<ref>{{cite journal | author = Singer W, Gray CM | title = Visual feature integration and the temporal correlation hypothesis | journal = Ann Rev Neurosci | volume = 18 | pages = 555–586 | year = 1995 | doi = 10.1146/annurev.ne.18.030195.003011 | pmid = 7605074}}</ref> According to this idea, synchronous oscillations in neuronal ensembles bind neurons representing different features of an object. For example, when a person looks at a tree, visual cortex neurons representing the tree trunk and those representing the branches of the same tree would oscillate in synchrony to form a single representation of the tree. This phenomenon is best seen in [[local field potentials]] which reflect the synchronous activity of local groups of neurons, but has also been shown in [[EEG]] and [[MEG]] recordings providing increasing evidence for a close relation between synchronous oscillatory activity and a variety of cognitive functions such as perceptual grouping.<ref name = "Singer 1993"/> |
||
| + | |||
| + | === Pacemaker === |
||
| + | {{main|Cardiac pacemaker}} |
||
| + | |||
| + | Cells in the [[sinoatrial node]], located in the [[right atrium]] of the heart, spontaneously depolarize approximately 100 times per minute. Although all of the heart's cells have the ability to generate action potentials that trigger cardiac contraction, the sinoatrial node normally initiates it, simply because it generates impulses slightly faster than the other areas. Hence, these cells generate the normal [[sinus rhythm]] and are called pacemaker cells as they directly control the [[heart rate]]. In the absence of extrinsic neural and hormonal control, cells in the SA node will rhythmically discharge. The sinoatrial node is richly innervated by the [[autonomic nervous system]], which up or down regulates the spontaneous firing frequency of the pacemaker cells. |
||
| + | |||
| + | === Central pattern generator === |
||
| + | {{main|Central pattern generator}} |
||
| + | |||
| + | Synchronized firing of neurons also forms the basis of periodic motor commands for rhythmic movements. These rhythmic outputs are produced by a group of interacting neurons that form a network, called a [[central pattern generator]]. Central pattern generators are neuronal circuits that - when activated - can produce rhythmic motor patterns in the absence of sensory or descending inputs that carry specific timing information. Examples are [[walking]], [[control of respiration|breathing]], and [[aquatic locomotion|swimming]], <ref>{{cite journal | author = Marder E, Bucher D | title = Central pattern generators and the control of rhythmic movements| journal = Curr Biol | volume = 11 | pages = R986-R996 | year = 2001 | doi = 10.1016/S0960-9822(01)00581-4}}</ref> Most evidence for central pattern generators comes from lower animals, such as the [[lamprey]], but there is also evidence for spinal central pattern generators in humans.<ref>{{cite journal | author = Dimitrijevic MR, Gerasimenko Y, Pinter MM | title = Evidence for a spinal central pattern generator in humans| journal = Ann NY Acad Sci | volume = 860 | pages = 360–376 | year = 1998 | doi = 10.1111/j.1749-6632.1998.tb09062.x | pmid=9928325}}</ref> |
||
| + | |||
| + | === Information processing === |
||
| + | {{main|Neural coding}} |
||
| + | |||
| + | Neuronal spiking is generally considered the basis for information transfer in the brain. For such a transfer, information needs to be coded in a spiking pattern. Different types of coding schemes have been proposed, such as [[rate coding]] and [[temporal coding]]. |
||
| + | |||
| + | === Perception === |
||
| + | {{see also| Binding problem}} |
||
| + | |||
| + | Synchronization of neuronal firing may serve as a means to group spatially segregated neurons that respond to the same stimulus in order to bind these responses for further joint processing, i.e. to exploit temporal synchrony to encode relations. Purely theoretical formulations of the binding-by-synchrony hypothesis were proposed first,<ref>{{cite journal | author = Milner PM | title = A model for visual shape recognition | journal = Psychological Rev | volume = 81 | issue = 6| pages = 521–535 | year = 1974 }}</ref> but subsequently extensive experimental evidence has been reported supporting the potential role of synchrony as a relational code.<ref name = "Gray 1989">{{cite journal | author = Gray CM, König P, Engel AK, Singer W | title = Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties | journal = Nature | volume = 338 | pages = 334–337 | year = 1989 |doi=10.1038/338334a0 | issue=6213}}</ref> |
||
| + | |||
| + | The functional role of synchronized oscillatory activity in the brain was mainly established in experiments performed on awake kittens with multiple electrodes implanted in the visual cortex. These experiments showed that groups of spatially segregated neurons engage in synchronous oscillatory activity when activated by visual stimuli. The frequency of these oscillations was in the range of 40 Hz and differed from the periodic activation induced by the grating, suggesting that the oscillations and their synchronization were due to internal neuronal interactions.<ref name="Gray 1989"/> Similar findings were shown in parallel by the group of Eckhorn providing further evidence for the functional role of neural synchronization in feature binding.<ref>{{cite journal | author = Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ | journal = Biol Cybern | volume = 60 | issue = 2 | title = Coherent oscillations: A mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat | year = 1988 | pages = 121–130 | doi= 10.1007/BF00202899 }}</ref> Since then numerous studies have replicated these findings and extended them to different modalities such as EEG, providing extensive evidence of the functional role of [[gamma wave|gamma]] oscillations in visual perception. |
||
| + | |||
| + | Gilles Laurent and colleagues showed that oscillatory synchronization has an important functional role in odor perception. Perceiving different odors leads to different subsets of neurons firing on different sets of oscillatory cycles.<ref>{{cite journal | author = Wehr M, Laurent G | title = Odour encoding by temporal sequences of firing in oscillating neural assemblies | journal = Nature | volume = 384 | pages = 162–166 | year = 1996 | doi = 10.1038/384162a0 | pmid = 8906790 | issue = 6605 }}</ref> These oscillations can be disrupted by [[GABA]] blocker [[picrotoxin]].<ref>{{cite journal | author = MacLeod K, Laurent G | title = Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies | journal = Science | volume = 274 | pages = 976–979 | year = 1996 | doi = 10.1126/science.274.5289.976 | pmid = 8875938 | issue = 5289 }}</ref> The disruption of the oscillatory synchronization leads to impairment of behavioral discrimination of chemically similar odorants in bees<ref>{{cite journal | author = Stopfer M, Bhagavan S, Smith BH, Laurent G | title = Impaired odour discrimination on desynchronization of odour-encoding neural assemblies | journal = Nature | volume = 390 | pages = 70–74 | year = 1997 | doi = 10.1038/36335 | pmid = 9363891 | issue = 6655 }}</ref> and to more similar responses across odors in downstream β-lobe neurons.<ref>{{cite journal | author = MacLeod K, Bäcker A, Laurent G | title = Who reads temporal information contained across synchronized and oscillatory spike trains? | journal = Nature | volume = 395 | pages = 693–698 | year = 1998 | doi = 10.1038/27201 | pmid = 9790189 | issue = 6703 }}</ref> |
||
| + | |||
| + | Neural oscillations are also thought be involved in the [[sense of time]]<ref>{{cite journal | author = Buhusi CV, Meck WH | year = 2005 | title = What makes us tick? Functional and neural mechanisms of interval timing | journal = Nat Rev Neurosci | volume = 6 | pages = 755–65 | doi = 10.1038/nrn1764 | pmid = 16163383 | issue = 10}}</ref> and in somatosensory perception.<ref>{{cite journal | author = Ahissar E, Zacksenhouse M | year = 2001 | title = Temporal and spatial coding in the rat vibrissal system | journal = Prog Brain Res | volume = 130 | pages = 75–87 | doi = 10.1016/S0079-6123(01)30007-9 | pmid = 11480290}}</ref> However, recent findings argue against a clock-like function of cortical gamma oscillations.<ref>{{cite journal | author = Burns SP, Xing D, Shapley RM | year = 2011 | title = Is gamma-band activity in the local field potential of V1 cortex a "clock" or filtered noise? | journal = J Neurosci | volume = 31 | pages = 9658–9664 | doi = 10.1523/jneurosci.0660-11.2011 | issue = 26}}</ref> |
||
| + | |||
| + | === Motor coordination === |
||
| + | {{main| Motor coordination}} |
||
| + | |||
| + | Oscillations have been commonly reported in the motor system. Pfurtscheller and colleagues found a reduction in [[alpha]] (8–12 Hz) and [[Beta wave|beta]] (13–30 Hz) oscillations in [[EEG]] activity when subjects made a movement.<ref name = "Pfurtscheller 1999"/><ref>{{cite journal | author = Pfurtscheller G, Aranibar A | title = Event-related cortical desynchronization detected by power measurements of scalp EEG | journal = Electroencephalogr Clin Neurophysiol | volume = 42 | pages = 817–826 | year = 1977 | pmid = 67933 | issue = 6}}</ref> Using intra-cortical recordings, similar changes in oscillatory activity were found in motor cortex when the monkeys performed motor acts that required significant attention.<ref>{{cite journal | author = Murthy VN, Fetz EE | title = Oscillatory activity in sensorimotor cortex of awake monkeys: Synchronization of local field potentials and relation to behavior | journal = J Neurophysiol | volume = 76 | pages = 3949–3967 | year = 1996 | pmid = 8985892 | issue = 6}}</ref><ref>{{cite journal | author = Sanes JN, Donoghue JP| title = Oscillations in local-field potentials of the primate motor cortex during voluntary movement | journal = PNAS | volume = 90 | pages = 4470–4474 | year = 1993 | doi = 10.1073/pnas.90.10.4470 | pmid = 8506287 | issue = 10 | pmc = 46533}}</ref> In addition, oscillations at spinal level become synchronised to beta oscillations in motor cortex during constant muscle activation, as determined by MEG/EEG-EMG coherence.<ref>{{cite journal | author = Conway, BA; Halliday, DM; Farmer, SF, et al.| title = Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man | journal = J Physiol | volume = 489 | pages = 917–924 | year = 1995 | issue = 3 }}</ref><ref>{{cite journal | author = Salenius S, Portin K, Kajola M, et al| title = Cortical control of human motoneuron firing during isometric contraction | journal = J Neurophysiol | volume = 77 | pages = 3401–3405 | year = 1997 | issue = 6 | pmid = 9212286}}</ref><ref>{{cite journal | doi = 10.1111/j.1469-7793.1997.225bo.x | author = Baker SN, Olivier E, Lemon RN | title = Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation | journal = J Physiol | volume = 501 | pages = 225–241 | year = 1997 | issue = 1 }}</ref> Recently it was found that cortical oscillations propagate as [[travelling wave]]s across the surface of the motor cortex along dominant spatial axes characteristic of the local circuitry of the motor cortex.<ref>{{cite journal | author = Rubino, D; Robbins, KA; Hatsopoulos, NG| title = Propagating waves mediate information transfer in the motor cortex | journal = Nat Neurosci | volume = 9 | pages = 1549–1557 | year = 2006 | doi = 10.1038/nn1802 | pmid = 17115042 | issue = 12}}</ref> |
||
| + | |||
| + | Oscillatory rhythms at 10 Hz have been recorded in a brain area called the [[inferior olive]], which is associated with the cerebellum.<ref name = "Llinas 1986"/> These oscillations are also observed in motor output of physiological [[tremor]]<ref>{{cite journal | author = Allum JHJ, Dietz V, Freund HJ| title = Neuronal mechanisms underlying physiological tremor | journal = J Neurophysiol | volume = 41 | pages = 557–571 | year = 1978 | pmid = 660226 | issue = 3}}</ref> and when performing slow finger movements.<ref>{{cite journal | author = Vallbo AB, Wessberg J| title = Organization of motor output of slow finger movements in man| journal = J Physiol | volume = 469 | pages = 673–691 | year = 1993 | pmid = 8271223 | pmc = 1143894}}</ref> These findings may indicate that the human brain controls continuous movements intermittently. In support, it was shown that these movement discontinuities are directly correlated to oscillatory activity in a cerebello-thalamo-cortical loop, which may represent a neural mechanism for the intermittent motor control.<ref>{{cite journal | author = Gross J, Timmermann J, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A | title = The neural basis of intermittent motor control in humans| journal = PNAS | volume = 99 | pages = 2299–2302 | year = 2002 | doi = 10.1073/pnas.032682099 | pmid = 11854526 | issue = 4 | pmc = 122359}}</ref> |
||
| + | |||
| + | === Memory === |
||
| + | {{main|Memory}} |
||
| + | |||
| + | Neural oscillations are extensively linked to memory function, in particular [[theta]] activity. Theta rhythms are very strong in rodent hippocampi and entorhinal cortex during learning and memory retrieval, and are believed to be vital to the induction of [[long-term potentiation]], a potential cellular mechanism of learning and memory. The coupling between theta and [[gamma]] activity is thought to be vital for memory functions.<ref>{{cite book | author = Buszaki G | title = Rhythms of the brain | year = 2006 | publisher = Oxford University Press}}</ref> The tight coordination of spike timing of single neurons with the local theta oscillations is linked to successful memory formation in humans, as more stereotyped spiking predicts better memory.<ref>{{cite journal | doi = 10.1038/nature08860 | author = Rutishauser U, Ross IB, Mamelak AN, Schuman EM | title = Human memory strength is predicted by theta-frequency phase-locking of single neurons | year = 2010 | journal = Nature | volume=464 | issue = 7290 | pages = 903–907 | pmid = 20336071}}</ref> |
||
| + | |||
| + | === Sleep and Consciousness === |
||
| + | {{main|Sleep}} |
||
| + | |||
| + | Sleep is a naturally recurring state characterized by reduced or absent [[consciousness]] and proceeds in cycles of [[rapid eye movement sleep|rapid eye movement]] (REM) and [[non-rapid eye movement sleep|non-rapid eye movement]] (NREM) sleep. The normal order of sleep stages is N1 → N2 → N3 → N2 → REM. Sleep stages are characterized by spectral content of EEG, for instance stage N1 refers to the transition of the brain from alpha waves (common in the awake state) to theta waves, whereas stage N3 (deep or slow-wave sleep) is characterized by the presence of delta waves. |
||
| + | |||
| + | == Pathology == |
||
| + | |||
| + | [[File:Writing by a Parkinson's disease patient.png|thumb|Handwriting of a person affected by [[Parkinson's disease]] showing rhythmic tremor activity in the strokes]] |
||
| + | [[File:Spike-waves.png|thumb|Generalized 3 Hz spike and wave discharges reflecting [[seizure]] activity]] |
||
| + | |||
| + | Specific types of neural oscillations may also appear in pathological situations, such as [[Parkinson's disease]] or [[epilepsy]]. Interestingly, these pathological oscillations often consist of an aberrant version of a normal oscillation. For example, one of the best known types is the [[spike and wave]] oscillation, which is typical of generalized or absence epileptic seizures, and which resembles normal sleep spindle oscillations. |
||
| + | |||
| + | === Tremor === |
||
| + | {{main|Tremor}} |
||
| + | |||
| + | A tremor is an involuntary, somewhat rhythmic, muscle contraction and relaxation involving to-and-fro movements of one or more body parts. It is the most common of all involuntary movements and can affect the hands, arms, eyes, face, head, vocal cords, trunk, and legs. Most tremors occur in the hands. In some people, tremor is a symptom of another neurological disorder. Many different forms of tremor have been identified, such as [[essential tremor]] or [[Parkinsonism|Parkinsonian]] tremor. It is argued that tremors are likely to be multifactorial in origin, with contributions from neural oscillations in the central nervous systems, but also from peripheral mechanisms such as reflex loop resonances.<ref>{{cite journal | author = McAuley JH, Marsden CD | title = Physiological and pathological tremors and rhythmic central motor control | journal = Brain | volume = 123 | year = 2000 | pages = 1545–1567}}</ref> |
||
| + | |||
| + | === Epilepsy === |
||
| + | {{main|Epilepsy}} |
||
| + | |||
| + | Epilepsy is a common chronic neurological disorder characterized by [[epileptic seizure|seizures]]. These seizures are transient signs and/or symptoms of abnormal, excessive or hypersynchronous neuronal activity in the brain. |
||
| + | {{clear}} |
||
| + | |||
| + | == Applications == |
||
| + | |||
| + | === Brain-computer interface === |
||
| + | {{main| Brain-computer interface}} |
||
| + | |||
| + | Neural oscillations have been considered for use as a control signal for various [[brain-computer interface]]s.<ref>{{cite journal | last=Birbaumer | first=Neils | year=2006 | title=Breaking the silence: Brain-computer interfaces (BCI) for communication and motor control | journal=Psychophysiology | pmid=17076808 | volume=43 | issue=6 | pages=517–32 | doi=10.1111/j.1469-8986.2006.00456.x}}</ref> A non-invasive BCI interface is created by placing electrodes on the scalp and then measuring the weak electric signals. Non-invasive BCI produces poor signal resolution because the skull dampens and blurs the electromagnetic signals. As a result, the activity of individual neurons can not be recovered, but oscillatory activity can still be reliably detected. In particular, some forms of BCI allow users to control a device by measuring the amplitude of oscillatory activity in specific frequency bands, including [[Mu wave|mu]] and [[Beta wave|beta]] rhythms. |
||
| + | {{-}} |
||
| + | |||
| + | == Examples == |
||
| + | |||
| + | A non-inclusive list of types of oscillatory activity found in the central nervous system: |
||
| + | *[[Delta wave]] |
||
| + | *[[Theta wave]] |
||
| + | *[[Alpha wave]] |
||
| + | *[[Mu wave]] |
||
| + | *[[Beta wave]] |
||
| + | *[[Gamma wave]] |
||
| + | *[[Sleep spindle]] |
||
| + | *[[Recurrent thalamo-cortical resonance|Thalamocortical oscillations]] |
||
| + | *[[Subthreshold membrane potential oscillations]] |
||
| + | *[[Bursting]] |
||
| + | *[[Cardiac cycle]] |
||
| + | *[[Epileptic seizure]] |
||
| + | |||
| + | == See also == |
||
| − | ==See also== |
||
| − | * [[Central pattern generator]] |
||
* [[Computational neuroscience]] |
* [[Computational neuroscience]] |
||
| + | * [[Systems neuroscience]] |
||
| + | * [[Neuro cybernetics]] |
||
* [[Cybernetics]] |
* [[Cybernetics]] |
||
* [[Dynamical systems theory]] |
* [[Dynamical systems theory]] |
||
* [[Electroencephalography]] |
* [[Electroencephalography]] |
||
* [[Magnetoencephalography]] |
* [[Magnetoencephalography]] |
||
| − | * [[Neuro cybernetics]] |
||
| − | ==References== |
+ | == References == |
{{Reflist|2}} |
{{Reflist|2}} |
||
| + | |||
| + | == Further reading == |
||
| + | |||
| + | *{{cite book | title=Rhythms of the Brain | last=Buzsáki | first=György | year=2006 | publisher=Oxford University Press | isbn=978-0-19-530106-9}} |
||
| + | |||
| + | == External links == |
||
| + | * [http://www.scholarpedia.org/article/Binding_by_synchrony Binding by synchronization] |
||
| + | * [http://www.scholarpedia.org/article/Neural_fields Neural Field Theory] |
||
| + | * [http://www.scholarpedia.org/article/Spike-and-wave_oscillations Spike-and-wave oscillations] |
||
| + | * [http://www.scholarpedia.org/article/Synchronization Synchronization] |
||
| + | * [http://www.scholarpedia.org/article/Bursting Bursting] |
||
| + | |||
| + | {{DEFAULTSORT:Neural Oscillation}} |
||
[[Category:Neuroscience]] |
[[Category:Neuroscience]] |
||
[[Category:Neural coding]] |
[[Category:Neural coding]] |
||
| Line 111: | Line 254: | ||
[[Category:Computational neuroscience]] |
[[Category:Computational neuroscience]] |
||
| + | [[ca:Oscil·lació neural]] |
||
| − | ==External links== |
||
| + | [[es:Onda cerebral]] |
||
| − | * [http://www.scholarpedia.org/article/Binding_by_synchrony Binding by synchronization] |
||
| + | [[fr:Oscillation neurale]] |
||
| − | * [http://www.scholarpedia.org/article/Neural_fields Neural Field Theory] |
||
| + | [[ru:Нейронные колебания]] |
||
| − | * [http://www.scholarpedia.org/article/Spike-and-wave_oscillations Spike-and-wave oscillations] |
||
| + | |||
| − | * [http://www.scholarpedia.org/article/Synchronization Synchronization] |
||
{{enWP|Neural oscillation}} |
{{enWP|Neural oscillation}} |
||
Revision as of 00:20, 7 May 2012
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
Neural oscillation is rhythmic or repetitive neural activity in the central nervous system. Neural tissue can generate oscillatory activity in many ways, driven either by mechanisms localized within individual neurons or by interactions between neurons. In individual neurons, oscillations can appear either as oscillations in membrane potential or as rhythmic patterns of action potentials, which then produce oscillatory activation of post-synaptic neurons. At the level of neural ensembles, synchronized activity of large numbers of neurons can give rise to macroscopic oscillations, which can be observed in the electroencephalogram (EEG). Oscillatory activity in groups of neurons generally arise from feedback connections between the neurons that result in the synchronization of their firing patterns. The interaction between neurons can give rise to oscillations at a different frequency than the firing frequency of individual neurons. A well-known example of macroscopic neural oscillations is alpha activity.
Neural oscillations were observed by researchers as early as Hans Berger, but their functional role is still not fully understood. The possible roles of neural oscillations include feature binding, information transfer mechanisms and the generation of rhythmic motor output. Over the last decades more insight has been gained, especially with advances in brain imaging. A major area of research in neuroscience involves determining how oscillations are generated and what their roles are. Oscillatory activity in the brain is widely observed at different levels of observation and is thought to play a key role in processing neural information. Numerous experimental studies indeed support a functional role of neural oscillations; a unified interpretation, however, is still lacking.
Simulation of neural oscillations at 10 Hz. Upper panel shows spiking of individual neurons (with each dot representing an individual action potential within the population of neurons), and the lower panel the local field potential reflecting their summed activity. Figure illustrates how synchronized patterns of action potentials may result in macroscopic oscillations that can be measured outside the scalp.
Overview
Neural oscillations are observed throughout the central nervous system and at all levels, e.g., spike trains, local field potentials and large-scale oscillations which can be measured by electroencephalography. In general, oscillations can be characterized by their frequency, amplitude and phase. These signal properties can be extracted from neural recordings using time-frequency analysis. In large-scale oscillations, amplitude changes are considered to result from changes in synchronization within a neural ensemble, also referred to as local synchronization. In addition to local synchronization, oscillatory activity of distant neural structures (single neurons or neural ensembles) can synchronize. Neural oscillations and synchronization have been linked to many cognitive functions such as information transfer, perception, motor control and memory.[1][2][3]
Neural oscillations have been most widely studied in neural activity generated by large groups of neurons. Large-scale activity can measured by techniques such as electroencephalography (EEG). In general, EEG signals have a broad spectral content similar to pink noise, but also reveal oscillatory activity in specific frequency bands. The first discovered and best-known frequency band is alpha activity (8–12 Hz) that can be detected from the occipital lobe during relaxed wakefulness and increases when the eyes are closed.[4] Other frequency bands are: delta (1–4 Hz), theta (4–8 Hz), beta (13–30 Hz) and gamma (30–70 Hz) frequency band, where faster rhythms such as gamma activity have been linked to cognitive processing. Indeed, EEG signals change dramatically during sleep and show a transition from faster frequencies such as alpha waves to increasingly slower frequencies. In fact, different sleep stages are commonly characterized by their spectral content.[5] Consequently, neural oscillations have been linked to cognitive states, such as awareness and consciousness.[6][7]
Although neural oscillations in human brain activity are mostly investigated using EEG recordings, they are also observed using more invasive recording techniques such as single-unit recordings. Neurons can generate rhythmic patterns of action potentials or spikes. Some types of neurons have the tendency to fire at particular frequencies, so-called resonators.[8] Bursting is another form of rhythmic spiking. Spiking patterns are considered fundamental for information coding in the brain. Oscillatory activity can also be observed in the form of subthreshold membrane potential oscillations (i.e. in the absence of action potentials).[9] If numerous neuron spike in synchrony, they can give rise to oscillations in local field potentials (LFPs). Quantitative models can estimate the strength of neural oscillation in recorded data.[10]
Neural oscillations are commonly studied from a mathematical framework and belong to the field of “neurodynamics”, an area of research in the cognitive sciences that places a strong focus upon the dynamic character of neural activity in describing brain function.[11]. It considers the brain a dynamical system and uses differential equations to describe how neural activity evolves over time. In particular, it aims to relate dynamic patterns of brain activity to cognitive functions such as perception and memory. In very abstract form, neural oscillations can be analyzed analytically. When studied in a more physiologically realistic setting, oscillatory activity is generally studied using computer simulations of a computational model.
The functions of neural oscillations are wide ranging and vary for different types of oscillatory activity. Examples are the generation of rhythmic activity such as a heartbeat and the neural binding of sensory features in perception, such as the shape and color of an object. Neural oscillations also play an important role in many neurological disorders, such as excessive synchronization during seizure activity in epilepsy or tremor in patients with Parkinson's disease. Oscillatory activity can also be used to control external devises in brain-computer interfaces, in which subjects can control an external device by changing the amplitude of particular brain rhythmics.
Physiology
- Main article: Electrophysiology
Oscillatory activity is observed throughout the central nervous system at all levels of organization. Three different levels have been widely recognized: the micro-scale (activity of a single neuron), the meso-scale (activity of a local group of neurons) and the macro-scale (activity of different brain regions).[12]
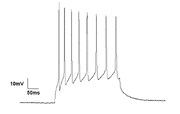
Tonic firing pattern of single neuron showing rhythmic spiking activity
Microscopic
Neurons generate action potentials resulting from changes in the electric membrane potential. Neurons can generate multiple action potentials in sequence forming so-called spike trains. These spike trains are the basis for neural coding and information transfer in the brain. Spike trains can form all kinds of patterns, such as rhythmic spiking and bursting, and often display oscillatory activity.[13] Oscillatory activity in single neurons can also be observed in sub-threshold fluctuations in membrane potential. These rhythmic changes in membrane potential do not reach the critical threshold and therefore do not result in an action potential. They can result from postsynaptic potentials from synchronous inputs or from intrinsic properties of neurons.
Neuronal spiking can be classified by their activity patterns. The excitability of neurons can be subdivided in Class I and II. Class I neurons can generate action potentials with arbitrarily low frequency depending on the input strength, whereas Class II neurons generate action potentials in a certain frequency band, which is relatively insensitive to changes in input strength.[8] Class II neurons are also more prone to display sub-threshold oscillations in membrane potential.
Mesoscopic
A group of neurons can also generate oscillatory activity. Through synaptic interactions the firing patterns of different neurons may become synchronized and the rhythmic changes in electric potential caused by their action potentials will add up (constructive interference). That is, synchronized firing patterns result in synchronised input into other cortical areas, which gives rise to large-amplitude oscillations of the local field potential. These large-scale oscillations can also be measured outside the scalp using electroencephalography and magnetoencephalography. The electric potentials generated by single neurons are far too small to be picked outside the scalp and EEG or MEG activity always reflects the summation of the synchronous activity of thousands or millions of neurons that have similar spatial orientation.[14] Neurons in a neural ensemble rarely fire all at the exact same moment, i.e. fully synchronized. Instead, the probability of firing is rhythmically modulated such that neurons are more likely to fire at the same time, which gives rise to oscillations in their mean activity (see figure at top of page). As such, the frequency of large-scale oscillations does not need to match the firing pattern of individual neurons. Isolated cortical neurons fire regularly under certain conditions, but in the intact brain cortical cells are bombarded by highly fluctuating synaptic inputs and typically fire seemingly random. However, if the probability of a large group of neurons is rhythmically modulated at a common frequency, they will generate oscillations in the mean field (see also figure at top of page).[13] Neural ensembles can generate oscillatory activity endogenously through local interactions between excitatory and inhibitory neurons. In particular, inhibitory interneurons play an important role in producing neural ensemble synchrony by generating a narrow window for effective excitation and rhythmically modulating the firing rate of excitatory neurons.[15]
Macroscopic
Neural oscillation can also arise from interactions between different brain areas. Time delays play an important role here. Because all brain areas are bidirectionally coupled, these connections between brain areas form feedback loops. Positive feedback loops tends to cause oscillatory activity which frequency is inversely related to the delay time. An example of such a feedback loop is the connections between the thalamus and cortex. This thalamocortical network is able to generate oscillatory activity known as recurrent thalamo-cortical resonance.[16] The thalamocortical network plays an important role in the generation of alpha activity.[17][18]
Mechanisms
Neuronal properties
- See also: Action potential and Bursting
Scientists have identified some intrinsic neuronal properties that play an important role in generating membrane potential oscillations. In particular, voltage-gated ion channels are critical in the generation of action potentials. The dynamics of these ion channels have been captured in the well-established Hodgkin-Huxley model that describes how action potentials are initiated and propagated by means of a set of differential equations. Using bifurcation analysis, different oscillatory varieties of these neuronal models can be determined, allowing for the classification of types of neuronal responses. The oscillatory dynamics of neuronal spiking as identified in the Hodgkin-Huxley model closely agree with empirical findings. In addition to periodic spiking, subthreshold membrane potential oscillations, i.e. resonance behavior that does not result in action potentials, may also contribute to oscillatory activity by facilitating synchronous activity of neighboring neurons.[19][20] Like pacemaker neurons in central pattern generators, subtypes of cortical cells fire bursts of spikes (brief clusters of spikes) rhythmically at preferred frequencies. Bursting neurons have the potential to serve as pacemakers for synchronous network oscillations, and bursts of spikes may underlie or enhance neuronal resonance.[13]
Network properties
- See also: Connectome and Oscillatory networks
Apart from intrinsic properties of neurons, network properties are also an important source of oscillatory activity. Neurons communicate with one another via synapses and affect the timing of spike trains in the post-synaptic neurons. Depending on the properties of the connection, such as the coupling strength, time delay and whether coupling is excitatory or inhibitory, the spike trains of the interacting neurons may become synchronized.[21] Neurons are locally connected, forming small clusters that are called neural ensembles. Certain network structures promote oscillatory activity at specific frequencies. For example, neuronal activity generated by two populations of interconnected inhibitory and excitatory cells can show spontaneous oscillations that are described by the Wilson-Cowan model.
If a group of neurons engages in synchronized oscillatory activity, the neural ensemble can be mathematically represented as a single oscillator.[12] Different neural ensembles are coupled through long-range connections and form a network of weakly coupled oscillators at the next spatial scale. Weakly coupled oscillators can generate a range of dynamics including oscillatory activity.[22] Long-range connections between different brain structures, such as the thalamus and the cortex (see thalamocortical oscillation), involve time-delays due to the finite conduction velocity of axons. Because most connections are reciprocal, they form feed-back loops that support oscillatory activity. Oscillations recorded from multiple cortical areas can become synchronized and form a large-scale network, whose dynamics and functional connectivity can be studied by means of spectral analysis and Granger causality measures.[23] Coherent activity of large-scale brain activity may form dynamic links between brain areas required for the integration of distributed information.[7]
Neuromodulation
In addition to fast direct synaptic interactions between neurons forming a network, oscillatory activity is modulated by neurotransmitters on a much slower time scale. That is, the concentration levels of certain neurotransmitters are known to regulate the amount of oscillatory activity. For instance, GABA concentration has been shown to be positively correlated with frequency of oscillations in induced stimuli.[24] A number of nuclei in the brainstem have diffuse projections throughout the brain influencing concentration levels of neurotransmitters such as norepinephrine, acetylcholine and serotonin. These neurotransmitter systems affect the physiological state, e.g., wakefulness or arousal, and have a pronounced effect on amplitude of different brain waves, such as alpha activity.[25]
Mathematical description
- See also: Computational neuroscience
Oscillations can often be described and analyzed using mathematics. Mathematicians have identified several dynamical mechanisms that generate rhythmicity. Among the most important are harmonic (linear) oscillators, limit-cycle oscillators, and delayed-feedback oscillators.[26] Harmonic oscillations appear very frequently in nature—examples are sound waves, the motion of a pendulum, and vibrations of every sort. They generally arise when a physical system is perturbed by a small degree from a minimum-energy state, and are well-understood mathematically. Noise-driven harmonic oscillators realistically simulate alpha rhythm in the waking EEG as well as slow waves and spindles in the sleep EEG. Successful EEG analysis algorithms were based on such models. Several other EEG components are better described by limit-cycle or delayed-feedback oscillations. Limit-cycle oscillations arise from physical systems that show large deviations from equilibrium, whereas delayed-feedback oscillations arise when components of a system affect each other after significant time delays. Limit-cycle oscillations can be complex but there are powerful mathematical tools for analyzing them; the mathematics of delayed-feedback oscillations is primitive in comparison. Linear oscillators and limit-cycle oscillators qualitatively differ in terms of how they respond to fluctuations in input. In a linear oscillator, the frequency is more or less constant but the amplitude can vary greatly. In a limit-cycle oscillator, the amplitude tends to be more or less constant but the frequency can vary greatly. A heartbeat is an example of a limit-cycle oscillation in that the frequency of beats varies widely, while each individual beat continues to pump about the same amount of blood.
Computational models adopt a variety of abstractions in order to describe complex oscillatory dynamics observed in brain activity. Many models are used in the field, each defined at a different level of abstraction and trying to model different aspects of neural systems. They range from models of the short-term behaviour of individual neurons, through models of how the dynamics of neural circuitry arise from interactions between individual neurons, to models of how behaviour can arise from abstract neural modules that represent complete subsystems.
Single neuron model
Simulation of a Hindmarsh-Rose neuron showing typical bursting behavior: a fast rhythm generated by individual spikes and a slower rhythm generated by the bursts.
- See also: Biological neuron model
A model of a biological neuron is a mathematical description of the properties of nerve cells, or neurons, that is designed to accurately describe and predict its biological processes. The most successful and widely-used model of neurons, the Hodgkin-Huxley model, is based on data from the squid giant axon. It is a set of nonlinear ordinary differential equations that approximates the electrical characteristics of a neuron, in particular the generation and propagation of action potentials. The model is very accurate and detailed and Hodgkin and Huxley received the 1963 Nobel Prize in physiology or medicine for this work.
The mathematics of the Hodgkin-Huxley model are quite complicated and several simplifications have been proposed, such as the FitzHugh-Nagumo model and the Hindmarsh-Rose model. Such models only capture the basic neuronal dynamics, such as rhythmic spiking and bursting, but are more computationally efficient. This allows the simulation of a large number of interconnected neurons that form a neural network.
Spiking model
- See also: Neural network
A neural network model describes a population of physically interconnected neurons or a group of disparate neurons whose inputs or signalling targets define a recognizable circuit. These models aim to describe how the dynamics of neural circuitry arise from interactions between individual neurons. Local interactions between neurons can result in the synchronization of spiking activity and form the basis of oscillatory activity. In particular, models of interacting pyramidal cells and inhibitory interneurons have been shown to generate brain rhythms such as gamma activity.[27]
Neural mass model
Simulation of a neural mass model showing network spiking during the onset of a seizure.[28] As the gain A is increased the network starts to oscillate at 3Hz.
- See also: Wilson-Cowan model
Neural field models are another important tool in studying neural oscillations and are a mathematical framework describing evolution of variables such as mean firing rate in space and time. In modeling the activity of large numbers of neurons, the central idea is to take the density of neurons to the continuum limit, resulting in spatially continuous neural networks. Instead of modelling individual neurons, this approach approximates a group of neurons by its average properties and interactions. It is based on the mean field approach, an area of statistical physics that deals with large-scale systems. Models based on these principles have been used to provide mathematical descriptions of neural oscillations and EEG rhythms. They have for instance been used to investigate visual hallucinations.[29]
Kuramoto model
Simulation of Kuramoto model showing neural synchronization and oscillations in the mean field
- Main article: Kuramoto model
The Kuramoto model of coupled phase oscillators[30] is one of the most abstract and fundamental model used to investigate neural oscillations and sychronization. It captures the activity of a local system (e.g., a single neuron or neural ensemble) by its circular phase alone and hence ignores the amplitude of oscillations (amplitude is constant).[31] Interactions amongst these oscillators are introduced by a simple algebraic form (such as a sin function) and collectively generate a dynamical pattern at the global scale. The Kuramoto model is widely used to study oscillatory brain activity and several extensions have been proposed that increase its neurobiological plausibility, for instance by incorporating topological properties of local cortical connectivity.[32] In particular, it describes how the activity of a group of interactioning neurons can become synchronized and generate large-scale oscillations. Simulations using the Kuramoto model with realistic long-range cortical connectivity and time-delayed interactions reveal the emergence of slow patterned fluctuations that reproduce resting-state BOLD functional maps, which can be measured using fMRI.[33]
Activity patterns
Both single and groups of neurons can generate oscillatory activity spontaneously. In addition, they may show oscillatory responses to perceptual input or motor output. Some types of neurons will fire rhythmically in the absence of any synaptic input. Likewise, brain wide activity reveals oscillatory activity while subjects do not engage in any activity, so-called resting-state activity. These ongoing rhythms can change in different ways in response to perceptual input or motor output. Oscillatory activity may respond by increases or decreases in frequency and amplitude or show a temporary interruption, which is referred to as phase resetting. In addition, external activity may not interact with ongoing activity at all, resulting in an additive response.
| Oscillatory responses | |||||||||
| |||||||||
Ongoing activity
Spontaneous activity is brain activity in the absence of an explicit task, such as sensory input or motor output, and hence also referred to as resting-state activity. It is opposed to induced activity, i.e. brain activity that is induced by sensory stimuli or motor responses. The term ongoing brain activity is used in electroencephalography and magnetoencephalography for those signal components that are not associated with the processing of a stimulus or the occurrence of specific other events, such as moving a body part, i.e. events that do not form evoked potentials/evoked fields, or induced activity. Spontaneous activity is usually considered to be noise if one is interested in stimulus processing. However, spontaneous activity is considered to play a crucial role during brain development, such as in network formation and synaptogenesis. Spontaneous activity may be informative regarding the current mental state of the person (e.g. wakefulness, alertness) and is often used in sleep research. Certain types of oscillatory activity, such as alpha waves, are part of spontaneous activity. Statistical analysis of power fluctuations of alpha activity reveals a bimodal distribution, i.e. a high- and low-amplitude mode, and hence shows that resting-state activity does not just reflect a noise process.[34] In case of fMRI, spontaneous fluctuations in the Blood-oxygen-level dependent (BOLD) signal reveal correlation patterns that are linked to resting states networks, such as the default network.[35] The temporal evolution of resting state networks is correlated with fluctuations of oscillatory EEG activity in different frequency bands.[36]
Ongoing brain activity may also have an important role in perception, as it may interact with activity related to incoming stimuli. Indeed, EEG studies suggest that visual perception is dependent on both the phase and amplitude of cortical oscillations. For instance, the amplitude and phase of alpha activity at the moment of visual stimulation predicts whether a weak stimulus will be perceived by the subject.[37][38][39]
Frequency response
In response to input, a neuron or neuronal ensemble may change the frequency at which it oscillates. This is very common in single neurons where the firing rate depends on the summed activity it receives. This is referred to as rate coding. Frequency changes are also commonly observed in central pattern generators and directly relate to the speed of motor activities, such as step frequency in walking. Changes in frequency are not so common in oscillatory activity involving different brain areas, as the frequency of oscillatory activity is often related to the time delays between brain areas.
Amplitude response
Next to evoked activity, neural activity related to stimulus processing may result in induced activity. Induced activity refers to modulation in ongoing brain activity induced by processing of stimuli or movement preparation. Hence, they reflect an indirect response in contrast to evoked responses. A well-studied type of induced activity is amplitude change in oscillatory activity. For instance, gamma activity often increases during increased mental activity such as during object representation.[40] Because induced responses may have different phases across measurements and therefore would cancel out during averaging, they can only be obtained using time-frequency analysis. Induced activity generally reflects the activity of numerous neurons: amplitude changes in oscillatory activity are thought to arise from the synchronization of neural activity, for instance by synchronization of spike timing or membrane potential fluctuations of individual neurons. Increases in oscillatory activity are therefore often referred to as event-related synchronization, while decreases are referred to as event-related desynchronization [41]
Phase resetting
Another possibility is that input to a neuron or neuronal ensemble resets the phase of ongoing oscillations.[42] Phase resetting is very common in single neurons where spike timing is adjusted to neuronal input. For instance, a neuron may start to spike at a fixed delay in response to periodic input, which is referred to as phase locking.[8] Phase resetting may also occur at the level of neuronal ensembles when the phases of multiple neurons are adjusted simultaneously. Phase resetting of ongoing ensemble oscillations gives an alternative explanation for event-related potentials obtained by averaging multiple EEG trials with respect to the onset of a stimulus or event.[43] That is, if the phase of ongoing oscillations is reset to a fixed phase over multiple trials, oscillations will no longer average out but add up to give rise to an event-related potential. Moreover, phase resetting or phase locking is also fundamental for the synchronization of different neurons or different brain regions.[22][7] In this case the timing of spikes becomes phase locked to the activity of other neurons instead of to external input.
Additive response
- See also: evoked potential
The term evoked activity is used in electroencephalography and magnetoencephalography for responses in brain activity that are directly related to stimulus-related activity. Evoked potentials and event-related potentials are obtained from the electroencephalogram by stimulus-locked averaging, i.e. averaging different trials at fixed latencies around the presentation of a stimulus. As a consequence, those signal components that are the same in each single measurement are conserved and all others, i.e. ongoing or spontaneous activity, are averaged out. That is, event-related potentials only reflect oscillations in brain activity that are phase-locked to the stimulus or event. Evoked activity is often considered to be independent from ongoing brain activity although this is an ongoing debate.[44]
Function
Neural synchronization can be modulated by task constraints, such as attention, and is thought to play a role in feature binding,[45] neuronal communication,[1] and motor coordination.[3] Neuronal oscillations became a hot topic in neuroscience in the 1990s when the studies of the visual system of the brain by Gray, Singer and others appeared to support the neural binding hypothesis.[46] According to this idea, synchronous oscillations in neuronal ensembles bind neurons representing different features of an object. For example, when a person looks at a tree, visual cortex neurons representing the tree trunk and those representing the branches of the same tree would oscillate in synchrony to form a single representation of the tree. This phenomenon is best seen in local field potentials which reflect the synchronous activity of local groups of neurons, but has also been shown in EEG and MEG recordings providing increasing evidence for a close relation between synchronous oscillatory activity and a variety of cognitive functions such as perceptual grouping.[45]
Pacemaker
- Main article: Cardiac pacemaker
Cells in the sinoatrial node, located in the right atrium of the heart, spontaneously depolarize approximately 100 times per minute. Although all of the heart's cells have the ability to generate action potentials that trigger cardiac contraction, the sinoatrial node normally initiates it, simply because it generates impulses slightly faster than the other areas. Hence, these cells generate the normal sinus rhythm and are called pacemaker cells as they directly control the heart rate. In the absence of extrinsic neural and hormonal control, cells in the SA node will rhythmically discharge. The sinoatrial node is richly innervated by the autonomic nervous system, which up or down regulates the spontaneous firing frequency of the pacemaker cells.
Central pattern generator
- Main article: Central pattern generator
Synchronized firing of neurons also forms the basis of periodic motor commands for rhythmic movements. These rhythmic outputs are produced by a group of interacting neurons that form a network, called a central pattern generator. Central pattern generators are neuronal circuits that - when activated - can produce rhythmic motor patterns in the absence of sensory or descending inputs that carry specific timing information. Examples are walking, breathing, and swimming, [47] Most evidence for central pattern generators comes from lower animals, such as the lamprey, but there is also evidence for spinal central pattern generators in humans.[48]
Information processing
- Main article: Neural coding
Neuronal spiking is generally considered the basis for information transfer in the brain. For such a transfer, information needs to be coded in a spiking pattern. Different types of coding schemes have been proposed, such as rate coding and temporal coding.
Perception
- See also: Binding problem
Synchronization of neuronal firing may serve as a means to group spatially segregated neurons that respond to the same stimulus in order to bind these responses for further joint processing, i.e. to exploit temporal synchrony to encode relations. Purely theoretical formulations of the binding-by-synchrony hypothesis were proposed first,[49] but subsequently extensive experimental evidence has been reported supporting the potential role of synchrony as a relational code.[50]
The functional role of synchronized oscillatory activity in the brain was mainly established in experiments performed on awake kittens with multiple electrodes implanted in the visual cortex. These experiments showed that groups of spatially segregated neurons engage in synchronous oscillatory activity when activated by visual stimuli. The frequency of these oscillations was in the range of 40 Hz and differed from the periodic activation induced by the grating, suggesting that the oscillations and their synchronization were due to internal neuronal interactions.[50] Similar findings were shown in parallel by the group of Eckhorn providing further evidence for the functional role of neural synchronization in feature binding.[51] Since then numerous studies have replicated these findings and extended them to different modalities such as EEG, providing extensive evidence of the functional role of gamma oscillations in visual perception.
Gilles Laurent and colleagues showed that oscillatory synchronization has an important functional role in odor perception. Perceiving different odors leads to different subsets of neurons firing on different sets of oscillatory cycles.[52] These oscillations can be disrupted by GABA blocker picrotoxin.[53] The disruption of the oscillatory synchronization leads to impairment of behavioral discrimination of chemically similar odorants in bees[54] and to more similar responses across odors in downstream β-lobe neurons.[55]
Neural oscillations are also thought be involved in the sense of time[56] and in somatosensory perception.[57] However, recent findings argue against a clock-like function of cortical gamma oscillations.[58]
Motor coordination
- Main article: Motor coordination
Oscillations have been commonly reported in the motor system. Pfurtscheller and colleagues found a reduction in alpha (8–12 Hz) and beta (13–30 Hz) oscillations in EEG activity when subjects made a movement.[41][59] Using intra-cortical recordings, similar changes in oscillatory activity were found in motor cortex when the monkeys performed motor acts that required significant attention.[60][61] In addition, oscillations at spinal level become synchronised to beta oscillations in motor cortex during constant muscle activation, as determined by MEG/EEG-EMG coherence.[62][63][64] Recently it was found that cortical oscillations propagate as travelling waves across the surface of the motor cortex along dominant spatial axes characteristic of the local circuitry of the motor cortex.[65]
Oscillatory rhythms at 10 Hz have been recorded in a brain area called the inferior olive, which is associated with the cerebellum.[9] These oscillations are also observed in motor output of physiological tremor[66] and when performing slow finger movements.[67] These findings may indicate that the human brain controls continuous movements intermittently. In support, it was shown that these movement discontinuities are directly correlated to oscillatory activity in a cerebello-thalamo-cortical loop, which may represent a neural mechanism for the intermittent motor control.[68]
Memory
- Main article: Memory
Neural oscillations are extensively linked to memory function, in particular theta activity. Theta rhythms are very strong in rodent hippocampi and entorhinal cortex during learning and memory retrieval, and are believed to be vital to the induction of long-term potentiation, a potential cellular mechanism of learning and memory. The coupling between theta and gamma activity is thought to be vital for memory functions.[69] The tight coordination of spike timing of single neurons with the local theta oscillations is linked to successful memory formation in humans, as more stereotyped spiking predicts better memory.[70]
Sleep and Consciousness
- Main article: Sleep
Sleep is a naturally recurring state characterized by reduced or absent consciousness and proceeds in cycles of rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. The normal order of sleep stages is N1 → N2 → N3 → N2 → REM. Sleep stages are characterized by spectral content of EEG, for instance stage N1 refers to the transition of the brain from alpha waves (common in the awake state) to theta waves, whereas stage N3 (deep or slow-wave sleep) is characterized by the presence of delta waves.
Pathology
Handwriting of a person affected by Parkinson's disease showing rhythmic tremor activity in the strokes
Generalized 3 Hz spike and wave discharges reflecting seizure activity
Specific types of neural oscillations may also appear in pathological situations, such as Parkinson's disease or epilepsy. Interestingly, these pathological oscillations often consist of an aberrant version of a normal oscillation. For example, one of the best known types is the spike and wave oscillation, which is typical of generalized or absence epileptic seizures, and which resembles normal sleep spindle oscillations.
Tremor
- Main article: Tremor
A tremor is an involuntary, somewhat rhythmic, muscle contraction and relaxation involving to-and-fro movements of one or more body parts. It is the most common of all involuntary movements and can affect the hands, arms, eyes, face, head, vocal cords, trunk, and legs. Most tremors occur in the hands. In some people, tremor is a symptom of another neurological disorder. Many different forms of tremor have been identified, such as essential tremor or Parkinsonian tremor. It is argued that tremors are likely to be multifactorial in origin, with contributions from neural oscillations in the central nervous systems, but also from peripheral mechanisms such as reflex loop resonances.[71]
Epilepsy
- Main article: Epilepsy
Epilepsy is a common chronic neurological disorder characterized by seizures. These seizures are transient signs and/or symptoms of abnormal, excessive or hypersynchronous neuronal activity in the brain.
Applications
Brain-computer interface
- Main article: Brain-computer interface
Neural oscillations have been considered for use as a control signal for various brain-computer interfaces.[72] A non-invasive BCI interface is created by placing electrodes on the scalp and then measuring the weak electric signals. Non-invasive BCI produces poor signal resolution because the skull dampens and blurs the electromagnetic signals. As a result, the activity of individual neurons can not be recovered, but oscillatory activity can still be reliably detected. In particular, some forms of BCI allow users to control a device by measuring the amplitude of oscillatory activity in specific frequency bands, including mu and beta rhythms.
Examples
A non-inclusive list of types of oscillatory activity found in the central nervous system:
- Delta wave
- Theta wave
- Alpha wave
- Mu wave
- Beta wave
- Gamma wave
- Sleep spindle
- Thalamocortical oscillations
- Subthreshold membrane potential oscillations
- Bursting
- Cardiac cycle
- Epileptic seizure
See also
- Computational neuroscience
- Systems neuroscience
- Neuro cybernetics
- Cybernetics
- Dynamical systems theory
- Electroencephalography
- Magnetoencephalography
References
- ↑ 1.0 1.1 Fries P (2001). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. TICS 9: 474–480.
- ↑ Fell J, Axmacher N (2011). The role of phase synchronization in memory processes. Nat Rev Neurosci 12: 105–118.
- ↑ 3.0 3.1 Schnitzler A, Gross J (2005). Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6 (4): 285–296.
- ↑ Berger H (1929). Uber das Elektroenkephalogramm des Menschen. Arch Psychiat Nervenkr 87: 527–570.
- ↑ Dement W, Kleitman N (1957). Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming. Electroencephalogr Clin Neurophysiol 9 (4): 673–90.
- ↑ Engel AK, Singer W (2001). Temporal binding and the neural correlates of sensory awareness 5 (1): 16–25.
- ↑ 7.0 7.1 7.2 Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001). The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2 (4): 229–239.
- ↑ 8.0 8.1 8.2 Izhikevich EM (2007). Dynamical systems in neuroscience, Cambridge, Massachusetts: The MIT Press.
- ↑ 9.0 9.1 Llinas R, Yarom Y (1986). Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol 376: 163–182.
- ↑ Mureşan RC, Jurjuţ OF, Moca VV, Singer W, Nikolić D (2008). The Oscillation Score: An Efficient Method for Estimating Oscillation Strength in Neuronal Activity. Journal of Neurophysiology 99 (3): 1333–1353.
- ↑ Burrow T (1943). The neurodynamics of behavior. A phylobiological foreword. Philosophy of Science 10: 271–288.
- ↑ 12.0 12.1 Haken H (1996). Principles of brain functioning, Springer.
- ↑ 13.0 13.1 13.2 Wang XJ (2010). Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90: 1195–1268.
- ↑ Nunez PL, Srinivasan R (1981). Electric fields of the brain: The neurophysics of EEG, Oxford University Press.
- ↑ Cardin JA, Carlen M, Meletis K, Knoblich, U, Zhang F, Deisseroth K, Tsai LH, Moore CI (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459 (7247): 663-U63.
- ↑ Llinas, Rodolfo (1998). The neuronal basis for consciousness. Phil Trans R Soc Lond 353: 1841–1849.
- ↑ Bollimunta, Anil (2011). Neuronal Mechanisms and Attentional Modulation of Corticothalamic Alpha Oscillations. The Journal of Neuroscience 31 (13): 4935–4943.
- ↑ Suffczynski P, Kalitzin S, Pfurtscheller G, Lopes da Silva FH (2001). Computational model of thalamo-cortical networks: dynamical control of alpha rhythms in relation to focal attention. Int J Psychophysiol 43 (1): 25–40.
- ↑ Llinas RR (1988). The Intrinsic electrophysiological properties of mammalian neurons: A new insight into CNS function. Science 242 (4886): 1654–1664.
- ↑ Llinas RR, Grace AA, Yarom Y (1991). In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci USA 88 (3): 897–901.
- ↑ Zeitler M, Daffertshofer A, Gielen CCAM (2009). Asymmetry in pulse-coupled oscillators with delay. Phys Rev E 79 (6).
- ↑ 22.0 22.1 Pikovsky A, Rosenblum M, Kurths J (2001). Synchronization: a universal concept in nonlinear sciences, Cambridge University Press.
- ↑ Andrea Brovelli, Steven L. Bressler and their colleagues, 2004
- ↑ Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD (2009). Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Nat Acad Sci USA 106 (20): 8356–8361.
- ↑ Moruzzi G, Magoun HW (1949). Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455–473.
- ↑ Buzsaki G, Draguhn A (2004). Neuronal oscillations in cortical networks. Science 304 (5679): 1926–1929.
- ↑ Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH (2000). Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol 38: 315–336.
- ↑ Wendling F, Bellanger JJ, Bartolomei F, Chauvel P (2000). Relevance of nonlinear lumped-parameter models in the analysis of depth-EEG epileptic signals. Biol Cybern 83: 367–378.
- ↑ Bressloff PC, Cowan JD (2003) Spontaneous pattern formation in primary visual cortex. In: J Hogan, AR Krauskopf, M di Bernado, RE Wilson (Eds.), Nonlinear dynamics and chaos: where do we go from here?
- ↑ Kuramoto Y (1984). Chemical Oscillations, Waves, and Turbulence, Dover Publications.
- ↑ Ermentrout B (1994). An introduction to neural oscillators. In F Ventriglia (ed.), Neural Modeling and Neural Networks: 79–110.
- ↑ Breakspear M, Heitmann S, Daffertshofer A (2010). Generative models of cortical oscillations: Neurobiological implications of the Kuramoto model. Front Hum Neurosc 4.
- ↑ Cabral J, Hugues E, Sporns O, Deco G (2011). Role of local network oscillations in resting-state functional connectivity. Neuroimage.
- ↑ Freyer F, Aquino K, Robinson PA, Ritter P, Breakspear M (2009). Bistability and non-Gaussian fluctuations in spontaneous cortical activity. J Neurosci 29 (26): 8512–8524.
- ↑ Fox MD, Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Neurosci Rev 8 (9): 700–711.
- ↑ Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A (2003). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. PNAS 100 (19): 11053–11058.
- ↑ Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T (2009). To see or not to see: Prestimulus α phase predicts visual awareness. J Neurosci 29 (9): 2725–32.
- ↑ Busch NA, Dubois J, VanRullen R (2009). The phase of ongoing EEG oscillations predicts visual perception. J Neurosci 29 (24): 7869–76.
- ↑ van Dijk H, Schoffelen JM, Oostenveld R, Jensen O (2008). Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28 (8): 1816–1823.
- ↑ Tallon-Baudry C, Bertrand O (1999). Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3: 151–162.
- ↑ 41.0 41.1 Pfurtscheller G, da Silva FHL (1999). Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857.
- ↑ Tass PA (2007). Phase resetting in medicine and biology: stochastic modelling and data analysis, Berlin Heidelberg: Springer-Verlag.
- ↑ Mäkinen V, Tiitinen H, May P (2005). Auditory event-related responses are generated independently of ongoing brain activity. NeuroImage 24: 961–968.
- ↑ Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ (2002). Dynamic brain sources of visual evoked responses. Science 295: 690–694.
- ↑ 45.0 45.1 Singer W (1993). Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol 55: 349–374.
- ↑ Singer W, Gray CM (1995). Visual feature integration and the temporal correlation hypothesis. Ann Rev Neurosci 18: 555–586.
- ↑ Marder E, Bucher D (2001). Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986-R996.
- ↑ Dimitrijevic MR, Gerasimenko Y, Pinter MM (1998). Evidence for a spinal central pattern generator in humans. Ann NY Acad Sci 860: 360–376.
- ↑ Milner PM (1974). A model for visual shape recognition. Psychological Rev 81 (6): 521–535.
- ↑ 50.0 50.1 Gray CM, König P, Engel AK, Singer W (1989). Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338 (6213): 334–337.
- ↑ Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ (1988). Coherent oscillations: A mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern 60 (2): 121–130.
- ↑ Wehr M, Laurent G (1996). Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature 384 (6605): 162–166.
- ↑ MacLeod K, Laurent G (1996). Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science 274 (5289): 976–979.
- ↑ Stopfer M, Bhagavan S, Smith BH, Laurent G (1997). Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390 (6655): 70–74.
- ↑ MacLeod K, Bäcker A, Laurent G (1998). Who reads temporal information contained across synchronized and oscillatory spike trains?. Nature 395 (6703): 693–698.
- ↑ Buhusi CV, Meck WH (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6 (10): 755–65.
- ↑ Ahissar E, Zacksenhouse M (2001). Temporal and spatial coding in the rat vibrissal system. Prog Brain Res 130: 75–87.
- ↑ Burns SP, Xing D, Shapley RM (2011). Is gamma-band activity in the local field potential of V1 cortex a "clock" or filtered noise?. J Neurosci 31 (26): 9658–9664.
- ↑ Pfurtscheller G, Aranibar A (1977). Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol 42 (6): 817–826.
- ↑ Murthy VN, Fetz EE (1996). Oscillatory activity in sensorimotor cortex of awake monkeys: Synchronization of local field potentials and relation to behavior. J Neurophysiol 76 (6): 3949–3967.
- ↑ Sanes JN, Donoghue JP (1993). Oscillations in local-field potentials of the primate motor cortex during voluntary movement. PNAS 90 (10): 4470–4474.
- ↑ Conway, BA; Halliday, DM; Farmer, SF, et al. (1995). Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol 489 (3): 917–924.
- ↑ Salenius S, Portin K, Kajola M, et al (1997). Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol 77 (6): 3401–3405.
- ↑ Baker SN, Olivier E, Lemon RN (1997). Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol 501 (1): 225–241.
- ↑ Rubino, D; Robbins, KA; Hatsopoulos, NG (2006). Propagating waves mediate information transfer in the motor cortex. Nat Neurosci 9 (12): 1549–1557.
- ↑ Allum JHJ, Dietz V, Freund HJ (1978). Neuronal mechanisms underlying physiological tremor. J Neurophysiol 41 (3): 557–571.
- ↑ Vallbo AB, Wessberg J (1993). Organization of motor output of slow finger movements in man. J Physiol 469: 673–691.
- ↑ Gross J, Timmermann J, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A (2002). The neural basis of intermittent motor control in humans. PNAS 99 (4): 2299–2302.
- ↑ Buszaki G (2006). Rhythms of the brain, Oxford University Press.
- ↑ Rutishauser U, Ross IB, Mamelak AN, Schuman EM (2010). Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464 (7290): 903–907.
- ↑ McAuley JH, Marsden CD (2000). Physiological and pathological tremors and rhythmic central motor control. Brain 123: 1545–1567.
- ↑ Birbaumer, Neils (2006). Breaking the silence: Brain-computer interfaces (BCI) for communication and motor control. Psychophysiology 43 (6): 517–32.
Further reading
- Buzsáki, György (2006). Rhythms of the Brain, Oxford University Press.
External links
ca:Oscil·lació neural es:Onda cerebral fr:Oscillation neurale ru:Нейронные колебания
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |