No edit summary |
No edit summary |
||
| Line 28: | Line 28: | ||
|} |
|} |
||
| − | '''Muscarine''', '''<small>L</small>-(+)-muscarine''', or '''muscarin''' is a [[Secondary metabolite|natural product]] found in certain |
+ | '''Muscarine''', '''<small>L</small>-(+)-muscarine''', or '''muscarin''' is a [[Secondary metabolite|natural product]] found in certain mushrooms, particularly in ''Inocybe'' and ''Clitocybe'' species, such as the deadly ''Clitocybe dealbata''. It was first isolated from ''Amanita muscaria'' in 1869. It was the first [[parasympathomimetic]] substance ever studied and causes profound activation of the [[Peripheral nervous system|peripheral]] [[parasympathetic nervous system]] that may end in convulsions and death. Muscarine has no effects on the [[central nervous system]] because it does not cross the [[blood-brain barrier]] due to its [[Electric charge|positively charged]] (polar) [[nitrogen]] ion. |
Muscarine mimics the action of the [[neurotransmitter]] [[acetylcholine]] at [[metabotropic]] receptors that are also known as ''[[muscarinic acetylcholine receptor|muscarinic ]]'' acetylcholine receptors. |
Muscarine mimics the action of the [[neurotransmitter]] [[acetylcholine]] at [[metabotropic]] receptors that are also known as ''[[muscarinic acetylcholine receptor|muscarinic ]]'' acetylcholine receptors. |
||
Latest revision as of 16:17, 21 December 2007
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
| Muscarine | |
|---|---|
Amanita muscaria from which muscarine was isolated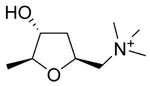
| |
| Systematic name | (2S,4R,5S)-(4-hydroxy-5-methyl- tetrahydrofuran-2-ylmethyl)- trimethyl-ammonium |
| Other names | L-(+)-muscarine, muscarin |
| Chemical formula | C9H20NO2+ |
| Molecular mass | 174.26 g/mol |
| CAS number | [300-54-9] |
| SMILES | O[C@@H]1C[C@@H](C[N+](C)(C)C)O[C@H]1C |
| Disclaimer and references | |
Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly Clitocybe dealbata. It was first isolated from Amanita muscaria in 1869. It was the first parasympathomimetic substance ever studied and causes profound activation of the peripheral parasympathetic nervous system that may end in convulsions and death. Muscarine has no effects on the central nervous system because it does not cross the blood-brain barrier due to its positively charged (polar) nitrogen ion.
Muscarine mimics the action of the neurotransmitter acetylcholine at metabotropic receptors that are also known as muscarinic acetylcholine receptors.
Muscarine poisoning is characterized by increased salivation, sweating (perspiration), and tearflow (lacrimation) within 15 to 30 minutes after ingestion of the mushroom. With large doses, these symptoms may be followed by abdominal pain, severe nausea, diarrhea, blurred vision, and labored breathing. Intoxication generally subsides within 2 hours. Death is rare, but may result from cardiac or respiratory failure in severe cases. The specific antidote is atropine.
Mushrooms in the genuses Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius, and Russula.
External links
References
- Katzung, Bertam G. Basic and Clinical Pharmacology, 9th ed. (2004). ISBN 0-07-141092-9
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |