m (Reverted edits by 71.105.21.235 (talk) to last version by Dr Joe Kiff) |
(update wp) |
||
| Line 1: | Line 1: | ||
| − | |||
{{ExpPsy}} |
{{ExpPsy}} |
||
{{Main|Postactivation potentials}} |
{{Main|Postactivation potentials}} |
||
| + | [[Image:LTP exemplar.jpg|thumb|300px|Long-term potentiation (LTP) is a persistent increase in [[synaptic strength]] following high-frequency stimulation of a [[chemical synapse]]. Studies of LTP are often carried out in slices of the [[hippocampus]], an important organ for learning and memory. In such studies, electrical recordings are made from cells and plotted in a graph such as this one. This graph compares the response to stimuli in synapses that have undergone LTP versus synapses that have not undergone LTP. Synapses that have undergone LTP tend to have stronger electrical responses to stimuli than other synapses. The term ''long-term potentiation'' comes from the fact that this increase in [[synaptic strength]], or ''potentiation'', lasts a very long time compared to other processes that affect synaptic strength.<ref name="isbn0-7817-6003-8">{{cite book |author=Paradiso, Michael A.; Bear, Mark F.; Connors, Barry W. |title=Neuroscience: Exploring the Brain |publisher=Lippincott Williams & Wilkins |location=Hagerstwon, MD |year=2007 |page=718 |isbn=0-7817-6003-8 |oclc= |doi= |accessdate=2008-12-25}}</ref>]] |
||
| − | [[Image:LTP_exemplar.jpg|thumb|300px|An example of long-term potentiation (LTP). The graph illustrates the analysis of a single field potential recording from a rat hippocampal slice. The inset shows the placement of the stimulating and recording electrode within the slice, and above, two raw traces of EPSP field potentials before and after tetanic stimulation. Field potentials were evoked by stimulation of Schaffer collaterals and recorded in stratum radiatum of the hippocampal slice (i.e. the Schaffer collateral/CA1 synapses). The individual points on the graph each represent the measurement of the rising slope of one EPSP field potential. Black squares indicate the measurements taken before tetanic stimluation. The green squares are measurements taken immediately after tetanic stimulation (PTP or post-tetanic potentiation) while the blue squares are measurments taken between 3 and 60 minutes after tetanization (LTP or long-term potentiation). Test stimuli were administered once every 30 sec. Tetanic stimulation was the test stimulus given at 100 Hz for 1 sec. Note how the amplitude of the EPSP field settles into a new, more elevated level after tetanic stimulation.]] |
||
| + | In [[neuroscience]], '''long-term potentiation''' ('''LTP''') is a long-lasting enhancement in signal transmission between two [[neuron]]s that results from stimulating them synchronously.<ref name="cooke">{{cite journal |author=Cooke SF, Bliss TV |title=Plasticity in the human central nervous system |journal=Brain |volume=129 |issue=Pt 7 |pages=1659–73 |year=2006 |pmid=16672292 |doi=10.1093/brain/awl082}}</ref> It is one of several phenomena underlying [[synaptic plasticity]], the ability of [[chemical synapse]]s to change their strength. As memories are thought to be encoded by modification of [[synaptic strength]],<ref name="bliss">{{cite journal |author=Bliss TV, Collingridge GL |title=A synaptic model of memory: long-term potentiation in the hippocampus |journal=Nature |volume=361 |issue=6407 |pages=31–9 |year=1993 |month=January |pmid=8421494 |doi=10.1038/361031a0 |url=http://dx.doi.org/10.1038/361031a0}}</ref> LTP is widely considered one of the major cellular mechanisms that underlies [[learning]] and [[memory]].<ref name="cooke"/><ref name="bliss"/> |
||
| − | In [[neuroscience]], '''long-term potentiation''' ('''LTP''') is the long-lasting strengthening of the connection between two [[neuron|nerve cells]]. Experimentally, a series of short, high-frequency electric stimulations to a nerve cell [[synapse]] can strengthen, or ''potentiate'', that synapse for minutes to hours. In living cells, LTP occurs naturally and can last from hours to days, months, and years. |
||
| + | LTP shares many features with [[long-term memory]], making it an attractive candidate for a cellular mechanism of learning. For example, LTP and long-term memory are triggered rapidly, each depends upon the [[protein biosynthesis|synthesis of new proteins]], each has properties of associativity, and each can last for many months.<ref name="cooke"/> LTP may account for many types of learning, from the relatively simple [[classical conditioning]] present in all animals, to the more complex, higher-level [[cognition]] observed in humans.<ref name="cooke"/> |
||
| − | The biological mechanisms of LTP, largely via the interplay of [[protein kinase]]s, [[phosphatase]]s, and [[gene expression]], give rise to [[synaptic plasticity]] and provide the foundation for a highly adaptable [[nervous system]]. Most neuroscientific [[learning theory|learning theories]] regard long-term potentiation and its opposing process, [[long-term depression]], as the cellular basis of [[learning]] and [[memory]]. |
||
| + | At a cellular level, LTP enhances [[synaptic transmission]]. It improves the ability of two neurons, one presynaptic and the other postsynaptic, to communicate with one another across a synapse. The precise molecular mechanisms for this enhancement of transmission have not been fully established, in part because LTP is governed by multiple mechanisms that vary by species and brain region. In the most well understood form of LTP, enhanced communication is predominantly carried out by improving the postsynaptic cell's sensitivity to signals received from the presynaptic cell.<ref name="riches"/> These signals, in the form of [[neurotransmitter]] molecules, are received by [[neurotransmitter receptor]]s present on the surface of the postsynaptic cell. LTP improves the postsynaptic cell's sensitivity to neurotransmitter in large part by increasing the activity of existing receptors and by increasing the number of receptors on the postsynaptic cell surface.<ref name="riches"/> |
||
| ⚫ | LTP was discovered in the |
||
| + | |||
| ⚫ | LTP was discovered in the rabbit [[hippocampus]] by [[Terje Lømo]] in 1966 and has remained a popular subject of research since. Many modern LTP studies seek to better understand its basic biology, while others aim to draw a causal link between LTP and behavioral learning. Still others try to develop methods, pharmacologic or otherwise, of enhancing LTP to improve learning and memory. LTP is also a subject of [[clinical research]], for example, in the areas of [[Alzheimer's disease]] and [[addiction medicine]]. |
||
==History== |
==History== |
||
===Early theories of learning=== |
===Early theories of learning=== |
||
| − | [[Image:Cajal.jpg|thumb|right| |
+ | [[Image:Cajal-mi.jpg|thumb|right|200px|The 19th century neuroanatomist [[Santiago Ramón y Cajal]] proposed that memories might be stored across [[synapse]]s, the junctions between neurons that allow for their communication.]] |
| + | At the end of the 19th century, scientists generally recognized that the number of neurons in the adult brain (roughly 100 billion<ref>{{cite journal |author=Williams RW, Herrup K |title=The control of neuron number |journal=Annu. Rev. Neurosci. |volume=11 |issue= |pages=423–53 |year=1988 |url=http://www.nervenet.org/papers/NUMBER_REV_1988.html |pmid=3284447 |doi=10.1146/annurev.ne.11.030188.002231}}</ref>) did not increase significantly with age, giving neurobiologists good reason to believe that memories were generally not the result of new neuron production.<ref name="cajal">{{cite journal | author = Ramón y Cajal, Santiago | title = The Croonian Lecture: La Fine Structure des Centres Nerveux | journal = Proceedings of the Royal Society of London | volume = 55 | pages = 444–468 | year = 1894 | doi = 10.1098/rspl.1894.0063 }}</ref> With this realization came the need to explain how memories could form in the absence of new neurons. |
||
| − | By about 1900, neurobiologists had good reason to believe that memories were generally not the product of new nerve cell growth. Scientists generally believed that the number of neurons in the adult brain (roughly 10<sup>11</sup>) did not increase significantly with age. With this realization came the need to explain how memories were created in the absence of new cell growth. |
||
| − | + | The [[Spain|Spanish]] neuroanatomist [[Santiago Ramón y Cajal]] was among the first to suggest a mechanism of learning that did not require the formation of new neurons. In his 1894 [[Croonian Lecture]], he proposed that memories might instead be formed by strengthening the connections between existing neurons to improve the effectiveness of their communication.<ref name="cajal"/> [[Hebbian theory]], introduced by [[Donald Olding Hebb|Donald Hebb]] in 1949, echoed Ramón y Cajal's ideas, further proposing that cells may grow new connections or undergo metabolic changes that enhance their ability to communicate: |
|
| − | + | {{quote|Let us assume that the persistence or repetition of a reverberatory activity (or "trace") tends to induce lasting cellular changes that add to its stability.... When an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A's efficiency, as one of the cells firing B, is increased.<ref>{{cite book | author=Hebb, D. O. | title=Organization of Behavior: a Neuropsychological Theory | location=New York | publisher=John Wiley | year=1949 | isbn=0-471-36727-3}}</ref>}} |
|
| − | + | Though these theories of memory formation are now well established, they were farsighted for their time: late 19th and early 20th century neuroscientists and psychologists were not equipped with the [[electrophysiology|neurophysiological]] techniques necessary for elucidating the biological underpinnings of learning in animals. These skills would not come until the latter half of the 20th century, at about the same time as the discovery of long-term potentiation. |
|
| − | ===Discovery |
+ | ===Discovery=== |
| − | [[Image:Hippocampus.png|thumb|right| |
+ | [[Image:Hippocampus.png|thumb|right|200px|LTP was first discovered in the rabbit [[hippocampus]]. In humans, the hippocampus is located in the medial [[temporal lobe]]. This illustration of the underside of the [[human brain]] shows the hippocampus highlighted in red. The [[frontal lobe]] is at the top of the illustration and the [[occipital lobe]] is at the bottom.]] |
| − | LTP was first observed by |
+ | LTP was first observed by Terje Lømo in 1966 in the [[Oslo]], [[Norway]], laboratory of [[Per Andersen]].<ref name="lomo">{{cite journal | author=Terje Lømo | title=The discovery of long-term potentiation | journal=Philos Trans R Soc Lond B Biol Sci | volume=358 | issue=1432 | year=2003 | pages=617–20 |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=12740104 |pmid=12740104 | doi=10.1098/rstb.2002.1226}}</ref><ref>{{cite journal |volume=68 |issue=Suppl 277 |pages=128 |last=Lømo |first=Terje |title=Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation |journal=Acta Physiologica Scandinavica |date=1966}}</ref> There, Lømo conducted a series of [[electrophysiology|neurophysiological]] experiments on [[anesthesia|anesthetized]] rabbits to explore the role of the hippocampus in [[short-term memory]]. |
| + | Lømo's experiments focused on connections, or synapses, from the [[perforant pathway]] to the [[dentate gyrus]]. These experiments were carried out by stimulating presynaptic fibers of the perforant pathway and recording responses from a collection of postsynaptic cells of the dentate gyrus. As expected, a single pulse of electrical stimulation to fibers of the perforant pathway caused [[excitatory postsynaptic potential]]s (EPSPs) in cells of the dentate gyrus. What Lømo unexpectedly observed was that the postsynaptic cells' response to these single-pulse stimuli could be enhanced for a long period of time if he first delivered a [[tetanic stimulation|high-frequency train of stimuli]] to the presynaptic fibers. When such a train of stimuli was applied, subsequent single-pulse stimuli elicited stronger, prolonged EPSPs in the postsynaptic cell population. This phenomenon, whereby a high-frequency stimulus could produce a long-lived enhancement in the postsynaptic cells' response to subsequent single-pulse stimuli, was initially called "long-lasting potentiation".<ref name="bliss_lomo">{{cite journal |author=Bliss T, Lømo T |title=Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path |journal=J Physiol |volume=232 |issue=2 |pages=331–56 |year=1973 |pmid=4727084 |url=http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1350458&blobtype=pdf}}</ref><ref name="bliss_g-m">{{cite journal |author=Bliss T, Gardner-Medwin A |title=Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path |journal=J. Physiol. (Lond.) |volume=232 |issue=2 |pages=357–74 |year=1973 |url=http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1350459&blobtype=pdf |pmid=4727085}}</ref> |
||
| − | Isolating the connections between two parts of the hippocampus, the [[perforant pathway]] and [[dentate gyrus]], Lømo observed the electrical changes in the dentate gyrus elicited by stimulation of the perforant pathway. As expected, a single pulse of electrical stimulation to the perforant pathway elicited an [[excitatory postsynaptic potential]] (EPSP) in the dentate gyrus. What Lømo did not expect was that a high-frequency train of stimulation produced larger, prolonged EPSPs compared to the responses evoked by a single stimulus. This phenomenon was soon dubbed "long-term potentiation". |
||
| + | Timothy Bliss, who joined the Andersen laboratory in 1968,<ref name="lomo"/> collaborated with Lømo and in 1973 the two published the first characterization of long-lasting potentiation in the [[rabbit]] hippocampus.<ref name="bliss_lomo"/> Bliss and Tony Gardner-Medwin published a similar report of long-lasting potentiation in the awake animal which appeared in the same issue as the Bliss and Lømo report.<ref name="bliss_g-m"/> In 1975, Douglas and Goddard proposed "long-term potentiation" as a new name for the phenomenon of long-lasting potentiation.<ref> While the term "long term potentiation" appeared once in the original Bliss and Lømo paper, it was not formally proposed for the phenomenon until the Douglas and Goddard paper.</ref><ref>{{cite journal |author=Douglas R, Goddard G |title=Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus |journal=Brain Res. |volume=86 |issue=2 |pages=205–15 |year=1975 |pmid=163667 |doi=10.1016/0006-8993(75)90697-6}}</ref> Andersen suggested that the authors chose "long-term potentiation" perhaps because of its easily pronounced acronym, "LTP".<ref>{{cite journal |author=Andersen P |title=A prelude to long-term potentiation |journal=Philos. Trans. R. Soc. Lond., B, Biol. Sci. |volume=358 |issue=1432 |pages=613–5 |year=2003 |url=http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1693144&blobtype=pdf |pmid=12740103 |doi=10.1098/rstb.2002.1232}}</ref> |
||
| − | Timothy Bliss, who joined the Andersen laboratory in [[1968]], collaborated with Lømo in [[1973]] to publish the first characterization of LTP in [[rabbit]] hippocampus.{{ref|blisslomo}} |
||
| − | ==Types |
+ | ==Types== |
| − | Since its original discovery in the rabbit hippocampus, LTP has been observed in a variety of other neural structures, including the [[cerebral cortex]], [[cerebellum]], [[amygdala]], |
+ | Since its original discovery in the rabbit hippocampus, LTP has been observed in a variety of other neural structures, including the [[cerebral cortex]], [[cerebellum]], [[amygdala]],<ref>{{cite journal |last=Clugnet |first=MC |coauthors=LeDoux JE |title=Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. |journal=J Neurosci |volume=10 |pages=2818–24 |pmid=2388089 |url=http://www.jneurosci.org/cgi/reprint/10/8/2818 |format=PDF |issue=8 |date=1 August 1990}}</ref> and many others. Robert Malenka, a prominent LTP researcher, has suggested that LTP may even occur at all excitatory synapses in the mammalian brain.<ref name="riches">{{cite journal |author=Malenka R, Bear M |title=LTP and LTD: an embarrassment of riches |journal=Neuron |volume=44 |issue=1 |pages=5–21 |year=2004 |pmid=15450156 |doi=10.1016/j.neuron.2004.09.012}}</ref> |
| + | Different areas of the brain exhibit different forms of LTP. The specific type of LTP exhibited between neurons depends on a number of factors. One such factor is the age of the organism when LTP is observed. For example, the molecular mechanisms of LTP in the immature hippocampus differ from those mechanisms that underlie LTP of the adult hippocampus.<ref name="developmental-switch">{{cite journal |author=Yasuda H, Barth A, Stellwagen D, Malenka R |title=A developmental switch in the signaling cascades for LTP induction |journal=Nat Neurosci |volume=6 |issue=1 |pages=15–6 |year=2003 |pmid=12469130 |doi=10.1038/nn985}}</ref> The signalling pathways used by a particular cell also contribute to the specific type of LTP present. For example, some types of hippocampal LTP depend on the [[NMDA receptor]], others may depend upon the [[metabotropic glutamate receptor]] (mGluR), while still others depend upon another molecule altogether.<ref name="riches"/> The variety of signaling pathways that contribute to LTP and the wide distribution of these various pathways in the brain are reasons that the type of LTP exhibited between neurons depends in part upon the anatomic location in which LTP is observed. For example, LTP in the [[Schaffer collateral]] pathway of the hippocampus is NMDA receptor-dependent, whereas LTP in the [[Mossy fiber (hippocampus)|mossy fiber]] pathway is NMDA receptor-independent.<ref>{{cite journal |author=Harris E, Cotman C |title=Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists |journal=Neurosci Lett |volume=70 |issue=1 |pages=132–7 |year=1986 |pmid=3022192 |doi=10.1016/0304-3940(86)90451-9}}</ref> |
||
| − | ===Associative LTP=== |
||
| − | Associative LTP is the molecular analog of associative learning (e.g. [[classical conditioning]]). When two or more synapses contribute to the firing (or repeated firings) of a neuron, then any resulting LTP affects all those "associated" synapses, regardless of each synapse's size of contribution. Conversely, synapses that are connected to the neuron but that do not contribute are not affected by LTP. |
||
| + | The pre- and postsynaptic activity required to induce LTP are other criteria by which LTP is classified. Broadly, this allows classification of LTP into Hebbian, non-Hebbian, and anti-Hebbian mechanisms. Borrowing its name from [[Hebb's postulate]], summarized by the maxim that "cells that fire together wire together," '''Hebbian LTP''' requires simultaneous pre- and postsynaptic depolarization for its induction.<ref name="pmid2883309">{{cite journal |author=Wigström H, Gustafsson B |title=Postsynaptic control of hippocampal long-term potentiation |journal=J. Physiol. (Paris) |volume=81 |issue=4 |pages=228–36 |year=1986 |pmid=2883309 |doi= |url=}}</ref> '''Non-Hebbian LTP''' is a type of LTP that does not require such simultaneous depolarization of pre- and postsynaptic cells; an example of this occurs in the mossy fiber hippocampal pathway.<ref name="pmid8753890">{{cite journal |author=Urban NN, Barrionuevo G |title=Induction of hebbian and non-hebbian mossy fiber long-term potentiation by distinct patterns of high-frequency stimulation |journal=J. Neurosci. |volume=16 |issue=13 |pages=4293–9 |year=1996 |month=July |pmid=8753890 |doi= |url=http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=8753890}}</ref> A special case of non-Hebbian LTP, '''anti-Hebbian LTP''' explicitly requires simultaneous presynaptic depolarization and relative postsynaptic hyperpolarization for its induction.<ref name="pmid18187472">{{cite journal |author=Kullmann DM, Lamsa K |title=Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation |journal=J. Physiol. (Lond.) |volume=586 |issue=6 |pages=1481–6 |year=2008 |month=March |pmid=18187472 |pmc=2375711 |doi=10.1113/jphysiol.2007.148064 |url=http://www.jphysiol.org/cgi/pmidlookup?view=long&pmid=18187472}}</ref> |
||
| − | To detect the simultaneous activity of synapses on a post-synaptic neuron, associative LTP requires a ''coincidence detector''. In many parts of the brain where associative LTP is observed, the [[NMDA receptor]] (NMDAR) fills the role of coincidence detector. At rest, the NMDAR's calcium channel is blocked by [[magnesium]]; the blockade is relieved only after strong postsynaptic depolarization{{ref|mayer-nmda_mgblock}}. The calcium channel is also [[ligand]]-gated, so that it only opens when presynaptically-released glutamate binds the receptor. When the NMDAR opens, calcium floods the postsynaptic cell, triggering associative LTP. |
||
| + | Owing to its predictable organization and readily inducible LTP, the CA1 hippocampus has become the prototypical site of mammalian LTP study. In particular, NMDA receptor-dependent LTP in the adult CA1 hippocampus is the most widely studied type of LTP,<ref name="riches"/> and is therefore the focus of this article. |
||
| − | NMDAR-dependent LTP has been demonstrated in the hippocampus, particularly in the Schaffer collaterals and perforant pathway, and several other brain regions including parts of the amygdala{{ref|rogan-fearcond}} and cerebral cortex{{ref|artola-visualcortex}}. |
||
| ⚫ | |||
| − | There are several types of associative LTP that do not depend on NMDA receptors. NMDAR-independent LTP has been observed, for example, in the amygdala, where it depends instead on [[voltage]]-gated calcium channels{{ref|weisskopf-ca_mediated_ltp}}. |
||
| ⚫ | |||
| + | ; Input specificity |
||
| − | ===Nonassociative LTP=== |
||
| + | : Once induced, LTP at one synapse does not spread to other synapses; rather LTP is ''input specific''. Long-term potentiation is only propagated to those synapses according to the rules of associativity and cooperativity. However, the input specificity of LTP may be incomplete at short distances. |
||
| − | Nonassociative LTP is brought about by the repeated application of one stimulus (whereas in associative LTP there are at least two stimuli). At nonassociative synapses, such as those involved in [[habituation]] and [[sensitization]], persistent stimulation of the synapse triggers an influx of calcium into the postsynaptic cell. As in associative LTP, calcium signals the beginning of long-term potentiation, but the precise mechanisms of nonassociative LTP are still unknown. |
||
| ⚫ | |||
| − | It is known that LTP requires both the release of glutamate as well as depolarisation of the postsynaptic cell. |
||
| ⚫ | |||
| + | ; Cooperativity |
||
| ⚫ | |||
| + | : LTP can be induced either by strong tetanic stimulation of a single pathway to a synapse, or ''cooperatively'' via the weaker stimulation of many. When one pathway into a synapse is stimulated weakly, it produces insufficient postsynaptic depolarization to induce LTP. In contrast, when weak stimuli are applied to many pathways that converge on a single patch of postsynaptic membrane, the individual postsynaptic depolarizations generated may collectively depolarize the postsynaptic cell enough to induce LTP cooperatively. Synaptic tagging, discussed later, may be a common mechanism underlying associativity and cooperativity. Bruce McNaughton argues that any difference between associativity and cooperativity is strictly semantic.<ref name="pmid12740107">{{cite journal |author=McNaughton BL |title=Long-term potentiation, cooperativity and Hebb's cell assemblies: a personal history |journal=Philosophical transactions of the Royal Society of London. Series B, Biological sciences |volume=358 |issue=1432 |pages=629–34 |year=2003 |month=April |pmid=12740107 |pmc=1693161 |doi=10.1098/rstb.2002.1231 |url=http://journals.royalsociety.org/openurl.asp?genre=article&issn=0962-8436&volume=358&issue=1432&spage=629}}</ref> |
||
| ⚫ | |||
| + | ; Persistence |
||
| ⚫ | |||
| + | : LTP is ''persistent'', lasting from several minutes to many months, and it is this persistence that separates LTP from other forms of [[synaptic plasticity]].<ref name="abraham">{{cite journal |author=Abraham WC |title=How long will long-term potentiation last? |journal=Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences |volume=358 |issue=1432 |pages=735–44 |year=2003 |month=April |pmid=12740120 |pmc=1693170 |doi=10.1098/rstb.2002.1222 |url=http://rstb.royalsocietypublishing.org/cgi/pmidlookup?view=long&pmid=12740120}}</ref> |
||
| − | LTP can be ''rapidly induced'' by applying one or more ''brief'' [[tetanic stimulation|tetanic stimuli]] to a presynaptic cell. (A tetanic stimulus is a high-frequency sequence of individual stimulation.) |
||
| − | == |
+ | ==Mechanism== |
| + | Long-term potentiation occurs through a variety of mechanisms throughout the nervous system; no single mechanism unites all of LTP's many types. However, for the purposes of study, LTP is commonly divided into three phases that occur sequentially: short-term potentiation, early LTP, and late LTP.<ref name="sweatt-kinases"/> Little is known about the mechanisms of short-term potentiation,<ref name="sweatt-kinases"/> so it will not be discussed here. |
||
| − | LTP can be induced either by strong tetanic stimulation of a single pathway to a synapse, or ''cooperatively'' via the weaker stimulation of many. It is explained by the presence of a stimulus threshold that must be reached in order to induce LTP. |
||
| + | Each phase of LTP is governed by a set of mediators, small molecules that dictate the events of that phase.<ref name="riches"/> These molecules include [[receptor (biochemistry)|protein receptors]] that respond to events outside of the cell, [[enzyme]]s that carry out [[chemical reaction]]s within the cell, and [[signaling molecule]]s that allow the progression from one phase to the next. In addition to these mediators, there are also modulator molecules, described later, that interact with mediators to finely alter the LTP ultimately generated. |
||
| − | When one pathway into a synapse is stimulated weakly, it produces insufficient postsynaptic depolarization to induce LTP. In contrast, when weak stimuli are applied to many pathways that converge on a single patch of the postsynaptic membrane, the individual postsynaptic depolarizations generated may collectively depolarize the postsynaptic cell enough to induce LTP cooperatively. |
||
| + | The early (E-LTP) and late (L-LTP) phases of LTP are each characterized by a series of three events: induction, maintenance, and expression. ''Induction'' is the process by which a short-lived signal triggers that phase of LTP to begin. ''Maintenance'' corresponds to the persistent biochemical changes that occur in response to the induction of that phase. ''Expression'' entails the long-lasting cellular changes that result from activation of the maintenance signal.<ref name="sweatt-kinases"/> Thus the mechanisms of LTP can be discussed in terms of the mediators that underlie the induction, maintenance, and expression of E-LTP and L-LTP. |
||
| ⚫ | |||
| ⚫ | ''Associativity'' refers to the observation that when weak stimulation of a single pathway is insufficient for the induction of LTP, simultaneous strong stimulation of another pathway will induce LTP at both pathways |
||
| − | === |
+ | ===Early phase=== |
| + | [[Image:Early LTP mechanism.png|right|thumb|200px|The early phase of LTP, one model of which is shown here, is independent of protein synthesis.<ref name="lynch"/>]] |
||
| − | Once induced, LTP at one synapse is not propagated to adjacent synapses; rather LTP is ''input specific''. |
||
| + | [[Image:CaMKII-dodecameric.png|thumb|200px|Ca<sup>2+</sup>/calmodulin-dependent protein kinase II (CaMKII) appears to be an important mediator of the early, protein synthesis-independent phase of LTP.]] |
||
| − | == |
+ | ====Induction==== |
| ⚫ | |||
| − | LTP is often divided into two phases, an early, protein synthesis-independent phase (E-LTP) that lasts between one and five hours, and a late, [[protein synthesis]]-dependent phase (L-LTP) that lasts from days to months{{ref|lu-nitricoxide}}. Broadly, E-LTP produces a potentiation of a few hours duration. It does so by making the postsynaptic side of the synapse more sensitive to glutamate by adding additional AMPA receptors into the postsynaptic membrane. |
||
| + | Induction of E-LTP occurs when the concentration of [[calcium]] inside the postsynaptic cell exceeds a critical threshold.<ref name="lynch">{{cite journal |author=Lynch M |title=Long-term potentiation and memory |journal=Physiol Rev |volume=84 |issue=1 |pages=87–136 |year=2004 |pmid=14715912 |url=http://physrev.physiology.org/cgi/content/full/84/1/87 |doi=10.1152/physrev.00014.2003}}</ref> In many types of LTP, the flow of calcium into the cell requires the [[NMDA receptor]], which is why these types of LTP are considered to be NMDA receptor-dependent.<ref name="lynch"/> NMDA receptor-dependent LTP can be induced experimentally by applying a few trains of high-frequency stimuli to the connection between two neurons.<ref>{{cite journal |author=Huang Y, Kandel E |title=Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization |journal=Learn Mem |volume=1 |issue=1 |pages=74–82 |year=1994 |pmid=10467587}}</ref> An understanding of normal synaptic transmission illustrates how this [[Tetany (action potential summation)|tetanic]] stimulation can induce E-LTP. |
||
| − | Conversely, L-LTP results in a pronounced strengthening of the postsynaptic response largely through the synthesis of new proteins. These proteins include glutamate receptors (e.g. AMPAR), [[transcription factor]]s, and structural proteins that enhance existing synapses and form new connections. There is also considerable evidence that late LTP prompts the postsynaptic synthesis of a retrograde messenger that diffuses to the presynaptic cell increasing the probability of neurotransmitter vesicle release on subsequent stimuli. All of this is largely hypothetical. The proposed mechanism of L-LTP are only weakly supported by existing data. Many investigators in the field doubt the very existence of L-LTP. |
||
| + | [[Chemical synapse]]s are functional connections between [[neuron]]s throughout the nervous system. In a typical synapse, information is passed from the first (presynaptic) neuron to the second (postsynaptic) neuron via a process of [[synaptic transmission]]. Through experimental manipulation, a non-tetanic stimulus can be applied to the presynaptic cell, causing it to release a [[neurotransmitter]]—typically [[glutamate]]—onto the postsynaptic cell membrane. There, glutamate binds to [[AMPA receptor]]s (AMPARs) embedded in the postsynaptic membrane. The AMPA receptor is one of the main excitatory receptors in the brain, and is responsible for most of its rapid, moment-to-moment excitatory activity.<ref>{{cite book |author=Agranoff, Bernard W.; Siegel, George J. |title=Basic neurochemistry: molecular, cellular, and medical aspects |publisher=Lippincott-Raven |location=Philadelphia |year=1999 |pages=326 |isbn=0-397-51820-X |oclc= |doi= |url=http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=bnchm.section.1131}}</ref> Glutamate binding to the AMPA receptor triggers the influx of positively charged [[sodium]] ions into the postsynaptic cell, causing a short-lived depolarization called the [[excitatory postsynaptic potential]] (EPSP). |
||
| − | ===Early LTP=== |
||
| − | E-LTP can be [[LTP induction|induced]] experimentally by applying a few trains of tetanic stimulation to the connection between two neurons{{ref|kandel-tetanization}}. Through normal [[synaptic transmission]], this stimulation causes the release of [[neurotransmitter]]s, particularly [[glutamate]], from the presynaptic terminal onto the postsynaptic cell membrane, where they bind to [[neurotransmitter receptor]]s embedded in the postsynaptic membrane. Though a single presentation of the stimulus is not sufficient to induce LTP, repeated presentations, if given at high-enough frequency, cause the postsynaptic cell to be progressively depolarized. This progressive depolarization is the result of [[EPSP]] summation. If each successive stimulus within a tetanic train reaches the postsynaptic cell before the previous EPSP can decay, successive EPSPs will add to the depolarization caused by the previous EPSPs. In synapses that exhibit NMDAR-dependent LTP, this progressive depolarization relieves the magnesium blockade of the NMDA receptor. When the magnesium block is removed, successive stimuli promote calcium entry through the NMDAR channel into the postsynaptic cell, rapidly increasing the intracellular concentration of calcium. It is this rapid rise in calcium concentration that induces E-LTP. |
||
| + | The magnitude of this depolarization determines whether E-LTP will be induced in the postsynaptic cell. While a single stimulus does not generate an EPSP capable of inducing E-LTP, repeated stimuli given at high frequency cause the postsynaptic cell to be progressively depolarized as a result of [[temporal summation|EPSP summation]]: with each EPSP reaching the postsynaptic cell before the previous EPSP can decay, successive EPSPs add to the depolarization caused by the previous EPSPs. In synapses that exhibit NMDA receptor-dependent LTP, sufficient depolarization unblocks [[NMDA receptor]]s (NMDARs), receptors that allow [[calcium]] to flow into the cell when bound by glutamate. While NMDARs are present at most postsynaptic membranes, at [[resting membrane potential]]s they are blocked by a [[magnesium]] ion that prevents the entry of calcium into the postsynaptic cell. Sufficient depolarization through the summation of EPSPs relieves the magnesium blockade of the NMDAR, allowing calcium influx (despite the reduced driving force for calcium entry). The rapid rise in intracellular calcium concentration triggers the short-lasting activation of several enzymes that mediate E-LTP induction. Of particular importance are some [[protein kinase]] enzymes, including [[calcium/calmodulin-dependent protein kinase II]] (CaMKII) and [[protein kinase C]] (PKC).<ref name="sweatt-kinases"/> To a lesser extent, [[protein kinase A]] (PKA) and [[mitogen-activated protein kinase]] (MAPK) activation also contribute to the induction of E-LTP.<ref name="sweatt-kinases"/> |
||
| − | Beyond calcium's critical role in the induction of E-LTP, few downstream molecular events leading to the expression and maintenance of E-LTP are known with certainty. Yet there is considerable evidence that E-LTP induction depends upon the activity of several [[protein kinase]]s, including [[calcium/calmodulin-dependent protein kinase II]] (CaMKII), [[protein kinase C]] (PKC) {{ref|kojima-fyn}}{{ref|malinow-pkc_camkii_inhib}}, [[protein kinase A]] (PKA){{ref|otmakhova-camp_inhibition}}, [[mitogen-activated protein kinase]] (MAPK){{ref|english-mapk_required}}{{ref|sweatt-mapk}}, and [[tyrosine kinase]]s{{ref|huang-tyrosinekinase}}. |
||
| + | ====Maintenance==== |
||
| − | Postsynaptically, the early phase of LTP is expressed primarily through the addition of new AMPA receptors to the postsynaptic membrane. In NMDA-dependent LTP in the CA1 hippocampus, the endogenous calcium [[chelator]] [[calmodulin]] rapidly binds calcium that is made available to it because it enters the cell through the NMDA receptor {{ref|malenka-calmodulin_pka}}. The calcium-calmodulin complex directly activates CaMKII which 1) phosphorylates voltage-gated potassium channels increasing their excitability{{ref|sweatt-ltp}}; 2) enhances the activity of existing AMPA receptors; and 3) phosphorylates intracellular AMPARs and activates Syn GAP (a [[Ras GTPase activating protein]]) and the MAPK cascade, facilitating the insertion of AMPARs into the postsynaptic membrane{{ref|esteban-ampa_trafficking}}. |
||
| + | While induction entails the ''transient'' activation of CaMKII and PKC, maintenance of E-LTP is characterized by their ''persistent'' activation. During this stage, PKMz([[Protein kinase Mζ]]) which does not have dependence on calcium, become autonomously active. Consequently they are able to carry out the phosphorylation events that underlie E-LTP expression.<ref name="sweatt-kinases"/> |
||
| + | ====Expression==== |
||
| − | PKA serves a role similar to that of CaMKII, but PKA's effects are more broad. PKA's activity is enhanced during LTP induction by elevated levels of cAMP as a result of calcium's activation of [[adenylyl cyclase]]-1<!--{{ref|otmakhova-camp_inhibition}}-->. Like CaMKII, PKA phosphorylates voltage-dependent potassium channels and also calcium channels enhancing their excitability to future stimuli. Additionally, PKA phosphorylates intracellular AMPAR stores, facilitating their insertion postsynaptically<!--{{ref|esteban-ampa_trafficking}}-->. PKA may also enhance AMPAR delivery via activation of the MAPK cascade<!--{{ref|sweatt-ltp}}-->. However, the role of PKA, especially in early LTP is very controversial. |
||
| + | [[Phosphorylation]] is a chemical reaction in which a small [[phosphate]] group is added to another molecule to change that molecule's activity. Autonomously active CaMKII and PKC use phosphorylation to carry out the two major mechanisms underlying the expression of E-LTP. First, and most importantly, they phosphorylate existing [[AMPA receptor]]s to increase their activity.<ref name="riches"/> Second, they mediate or modulate the insertion of additional AMPA receptors into the postsynaptic membrane.<ref name="riches"/> Importantly, the delivery of AMPA receptors to the synapse during E-LTP is independent of [[Protein biosynthesis|protein synthesis]]. This is achieved by having a nonsynaptic pool of AMPA receptors adjacent to the postsynaptic membrane. When the appropriate LTP-inducing stimulus arrives, nonsynaptic AMPA receptors are rapidly trafficked into the postsynaptic membrane under the influence of protein kinases.<ref>{{cite journal |author=Malinow R |title=AMPA receptor trafficking and long-term potentiation |journal=Philos Trans R Soc Lond B Biol Sci |volume=358 |issue=1432 |pages=707–14 |year=2003 |pmid=12740116 |doi=10.1098/rstb.2002.1233}}</ref> As mentioned previously, AMPA receptors are the brain's most abundant glutamate receptors and mediate the majority of its excitatory activity. By increasing the efficiency and number of AMPA receptors at the synapse, future excitatory stimuli generate larger postsynaptic responses. |
||
| + | While the above model of E-LTP describes entirely postsynaptic mechanisms for induction, maintenance, and expression, an additional component of expression may occur presynaptically.<ref name="emptage">{{cite journal |author=Emptage N, Reid C, Fine A, Bliss T |title=Optical quantal analysis reveals a presynaptic component of LTP at hippocampal Schaffer-associational synapses |journal=Neuron |volume=38 |issue=5 |pages=797–804 |year=2003 |pmid=12797963 |url=http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WSS-4C5XN9K-G&_coverDate=06%2F05%2F2003&_alid=523934887&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=7054&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=15f6a55d9b53d2265077f3a74a8a495a |doi=10.1016/S0896-6273(03)00325-8}}</ref> One hypothesis of this presynaptic facilitation is that persistent CaMKII activity during E-LTP may lead to the synthesis of a "retrograde messenger", discussed later. According to this hypothesis, the newly synthesized messenger travels across the synaptic cleft from the postsynaptic to the presynaptic cell, leading to a chain of events that facilitate the presynaptic response to subsequent stimuli. Such events may include an increase in neurotransmitter vesicle number, probability of vesicle release, or both. In addition to the retrograde messenger underlying presynaptic expression in early LTP, the retrograde messenger may also play a role in the expression of late LTP. |
||
| − | While LTP is induced postsynaptically, it is partially expressed presynaptically. One hypothesis of presynaptic facilitation is that enhanced CaMKII activity during early LTP gives rise to CaMKII autophosphorylation and constitutive activation. Persistent CaMKII activity then stimulates NO synthase, leading to the enhanced production of the putative retrograde messenger, NO. Since NO is a diffusable gas, it freely diffuses across the synaptic cleft to the presynaptic cell leading to a chain of molecular events that facilitate the presynaptic response to subsequent stimuli. (''See [[#Retrograde signaling|Retrograde signaling]] for discussion about the identity of the retrograde messenger.'') |
||
| − | ===Late |
+ | ===Late phase=== |
| − | [[Image: |
+ | [[Image:Late LTP mechanism.png|right|thumb|200px|The early and late phases of LTP are thought to communicate via the [[extracellular signal-regulated kinase]] (ERK).<ref name="lynch"/>]] |
| + | Late LTP is the natural extension of E-LTP. Unlike E-LTP, which is independent of protein synthesis, L-LTP requires [[gene transcription]]<ref>{{cite journal |author=Frey U, Frey S, Schollmeier F, Krug M |title=Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro |journal=J Physiol |volume=490 (Pt 3) |issue= Pt 3|pages=703–11 |date=1 January 1996|pmid=8683469 |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=8683469 }}</ref> and [[Protein biosynthesis|protein synthesis]]<ref>{{cite journal |author=Frey U, Krug M, Reymann K, Matthies H |title=Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro |journal=Brain Res |volume=452 |issue=1-2 |pages=57–65 |year=1988 |pmid=3401749 |doi=10.1016/0006-8993(88)90008-X}}</ref> in the postsynaptic cell. Two phases of L-LTP exist: the first depends upon protein synthesis, while the second depends upon both gene transcription and protein synthesis.<ref name="lynch"/> These phases are occasionally called LTP2 and LTP3, respectively, with E-LTP referred to as LTP1 under this nomenclature. |
||
| − | Late LTP can be experimentally induced by a series of three or more trains of tetanic stimulation spaced roughly 10 minutes apart. Unlike early LTP, late LTP requires gene transcription{{ref|nguyen-transcription}}{{ref|bourtchuladze-camp-mut}} and protein synthesis{{ref|frey-translation}}, making it an attractive candidate for the molecular analog of long-term memory<!--{{ref|bourtchuladze-camp-mut}}-->. |
||
| + | ====Induction==== |
||
| − | The synthesis of gene products is driven by kinases which in turn activate [[transcription factor]]s that mediate gene expression. [[cAMP response element binding protein]]-1 (CREB-1) is thought to be the primary transcription factor in the cascade of gene expression that leads to prolonged structural changes to the synapse enhancing its strength{{ref|poser-ac}}. CREB-1 is both necessary{{ref|dash-cre-inj}} and sufficient{{ref|bartsch-creb}} for late LTP. It is active in its phosphorylated form and induces the transcription of so-called ''immediate-early genes'', including [[c-fos]] and [[c-jun]]{{ref|kasahara-pkiv}}. Ultimately, the products of CREB-1-mediated transcription and protein synthesis give rise to new building materials for the synaptic connection between pre- and postsynaptic cell. |
||
| + | Late LTP is induced by changes in [[gene expression]] and [[Protein biosynthesis|protein synthesis]] brought about by the persistent activation of protein kinases activated during E-LTP, such as MAPK.<ref name="sweatt-kinases"/><ref name="lynch"/><ref name="translation"/> In fact, MAPK—specifically the [[extracellular signal-regulated kinase]] (ERK) subfamily of MAPKs—may be the molecular link between E-LTP and L-LTP, since many signaling cascades involved in E-LTP, including CaMKII and PKC, can converge on ERK.<ref name="translation"/> Recent research has shown that the induction of L-LTP can depend on coincident molecular events, namely PKA activation and calcium influx, that converge on CRTC1 (TORC1), a potent transcriptional coactivator for CREB.<ref> {{cite journal | author=Kovács KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR|title=TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. |journal=PNAS |volume=104 |issue= 11 |pages= 4700–5 |year= 2007 |pmid= 17360587 |doi= 10.1073/pnas.0607524104}}</ref> This requirement for a molecular coincidence accounts perfectly for the associative nature of LTP, and, presumably, for that of learning. |
||
| + | ====Maintenance==== |
||
| − | During L-LTP, constitutively active CaMKII activates a related kinase, CaMKIV. Additionally, enhanced Ca<sup>2+</sup> levels during late LTP increase cAMP synthesis via adenylyl cyclase-1, further activating PKA and resulting in the phosphorylation and activation of MAPK{{ref|huang-pka-mapk}}. Facilitated by cAMP, both CaMKII and CaMKIV translocate to the [[cell nucleus]] along with PKA and MAPK (mediated by PKA){{ref|impey-erk-pka}}, where they phosphorylate CREB-1{{ref|segal-creb}}. |
||
| + | Upon activation, ERK may phosphorylate a number of cytoplasmic and nuclear molecules that ultimately result in the protein synthesis and morphological changes observed in L-LTP.<ref name="lynch"/> These cytoplasmic and nuclear molecules may include [[transcription factor]]s such as [[cAMP response element binding protein]] (CREB).<ref name="sweatt-kinases"/> ERK-mediated changes in transcription factor activity may trigger the synthesis of proteins that underlie the maintenance of L-LTP. One such molecule may be [[protein kinase Mζ]] (PKMζ), a persistently active kinase whose synthesis increases following LTP induction.<ref name="PKMz">{{cite journal |author=Serrano P, Yao Y, Sacktor T |title=Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation |journal=J Neurosci |volume=25 |issue=8 |pages=1979–84 |year=2005 |pmid=15728837 |doi=10.1523/JNEUROSCI.5132-04.2005}}</ref><ref name="PKMz-maintenance">{{cite journal |author=Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton A, Sacktor T |title=Storage of spatial information by the maintenance mechanism of LTP |journal=Science |volume=313 |issue=5790 |pages=1141–4 |year=2006 |pmid=16931766 |doi=10.1126/science.1128657}}</ref> PKMζ is an atypical isoform of PKC that lacks a regulatory subunit and thus remains constitutively active.<ref name="PKMz"/> Unlike other kinases that mediate LTP, PKMζ is active not just in the first 30 minutes following LTP induction; rather, PKMζ becomes a requirement for LTP maintenance only during the late phase of LTP.<ref name="PKMz"/> PKMζ thus appears important for the persistence of memory and would be expected to be important in the maintenance of [[long-term memory]]. Indeed, administration of a PKMζ inhibitor into the hippocampi of rats results in [[retrograde amnesia]] with intact [[short-term memory]]; PKMζ does not play a role in the establishment of short-term memory.<ref name="PKMz-maintenance"/> PKMζ has recently been shown to underlie L-LTP maintenance<ref name="PKMz"/><ref name="PKMz-maintenance"/> by directing the trafficking and reorganization of proteins in the synaptic scaffolding that underlie the expression of L-LTP.<ref name="PKMz"/> |
||
| + | ====Expression==== |
||
| − | There is also some evidence that L-LTP is mediated in part by [[nitric oxide]] (NO){{ref|lu-no}}. In particular, NO may activate [[guanylyl cyclase]], leading to the production of [[cyclic GMP]] and activation [[protein kinase G]] (PKG), which phosphorylates CREB-1. PKG may also cause the release of Ca<sup>2+</sup> from [[ryanodine receptor]]-gated intracellular stores, increasing the Ca<sup>2+</sup> concentration which activates other previously mentioned kinase cascades to further activate CREB-1. |
||
| + | Aside from PKMζ, the identities of only a few proteins synthesized during L-LTP are known. Regardless of their identities, it is thought that they contribute to the increase in [[dendritic spine]] number, surface area, and postsynaptic sensitivity to neurotransmitter associated with L-LTP expression.<ref name="lynch"/> The latter may be brought about in part by the enhanced synthesis of AMPA receptors during L-LTP.<ref name="lynch"/> Late LTP is also associated with the presynaptic synthesis of [[synaptotagmin]] and an increase in [[synaptic vesicle]] number, suggesting that L-LTP induces protein synthesis not only in postsynaptic cells, but in presynaptic cells as well.<ref name="lynch"/> As mentioned previously, for postsynaptic LTP induction to result in presynaptic protein synthesis, there must be communication from the postsynaptic to the presynaptic cell. This may occur via the synthesis of a retrograde messenger, discussed later. |
||
| + | Even in studies restricted to postsynaptic events, investigators have not determined the location of the protein synthesis that underlies L-LTP. Specifically, it is unclear whether protein synthesis takes place in the postsynaptic [[cell body]] or in its [[dendrite]]s.<ref name="translation">{{cite journal |author=Kelleher R, Govindarajan A, Tonegawa S |title=Translational regulatory mechanisms in persistent forms of synaptic plasticity |journal=Neuron |volume=44 |issue=1 |pages=59–73 |year=2004 |pmid=15450160 |doi=10.1016/j.neuron.2004.09.013}}</ref> Despite having observed [[ribosome]]s (the major components of the protein synthesis machinery) in dendrites as early as the 1960s, prevailing wisdom was that the cell body was the predominant site of protein synthesis in neurons.<ref name="translation"/> This reasoning was not seriously challenged until the 1980s, when investigators reported observing protein synthesis in dendrites whose connection to their cell body had been severed.<ref name="translation"/> More recently, investigators have demonstrated that this type of local protein synthesis is necessary for some types of LTP.<ref>{{cite journal |author=Kang H, Schuman E |title=A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity |journal=Science |volume=273 |issue=5280 |pages=1402–6 |year=1996 |pmid=8703078 |doi=10.1126/science.273.5280.1402}}</ref><ref>{{cite journal |author=Steward O, Worley P |title=A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites |journal=Proc Natl Acad Sci USA |volume=98 |issue=13 |pages=7062–8 |year=2001 |pmid=11416188 |url=http://www.pnas.org/cgi/content/full/98/13/7062 |doi=10.1073/pnas.131146398}}</ref> |
||
| ⚫ | |||
| − | Retrograde signalling is a theoretical concept that arises from the question: "If LTP is induced postsynaptically, but expressed presynaptically, how does the presynaptic terminal "know" that LTP has been induced?" The obvious answer is that there must be some communication "backwards" across the synapse, that is, in the retrograde direction from the postsynaptic to the presynaptic side. This concept led to a flurry of work in the early 1990's to demonstrate the existence of a retrograde messenger and also to identify such a messenger. A number of candidates were examined including [[carbon monoxide]]{{ref|alkadhi-retro_co}}, [[platelet-activating factor]]{{ref|kato-retro_paf}}{{ref|kato-retro_paf2}}, arachidonic acid, and nitric oxide{{ref|arancio-retro_no}}{{ref|hawkins-retro_no}}. |
||
| + | One reason for the popularity of the local protein synthesis hypothesis is that it provides a possible mechanism for the specificity associated with LTP.<ref name="translation"/> Specifically, if indeed local protein synthesis underlies L-LTP, only dendritic spines receiving LTP-inducing stimuli will undergo LTP; the potentiation will not be propagated to adjacent synapses. By contrast, global protein synthesis that occurs in the cell body requires that proteins be shipped out to every area of the cell, including synapses that have not received LTP-inducing stimuli. Whereas local protein synthesis provides a mechanism for specificity, global protein synthesis would seem to directly compromise it. However, as discussed later, the synaptic tagging hypothesis successfully reconciles global protein synthesis, synapse specificity, and associativity. |
||
| − | Perhaps unfortunately for the retrograde signaling hypothesis, subsequent work has strongly established that LTP, at least early LTP, is expressed entirely postsynaptically (cf. Malenka and Bear, 2004). However, there is still life in the retrograde signalling hypothesis, since it has been demonstrated that ''induction'' of LTP may involve a retrograde messenger, since contrary to dogma, LTP induction does not appear to be entirely postsynaptic (Pavlidis, et al., 2000). |
||
| ⚫ | |||
| ⚫ | |||
| + | {{main|Retrograde signaling in LTP}} |
||
| − | The gene expression and protein synthesis that mediate the long-term changes of LTP generally take place in the cell body, but LTP is synapse-specific; LTP induced at one synapse does not propagate to adjacent inactive synapses. Therefore, the cell is posed with the difficult problem of synthesizing plasticity-related proteins in the nucleus and cell body, but ensuring they only reach synapses that have received LTP-inducing stimuli. |
||
| + | Retrograde signaling is a hypothesis that attempts to explain that, while LTP is induced and expressed postsynaptically, some evidence suggests that it is expressed presynaptically as well.<ref name="riches"/><ref name="emptage"/><ref>{{cite journal |author=Pavlidis P, Montgomery J, Madison D |title=Presynaptic protein kinase activity supports long-term potentiation at synapses between individual hippocampal neurons |journal=J Neurosci |volume=20 |issue=12 |pages=4497–505 |year=2000 |pmid=10844019}}</ref> The hypothesis gets its name because normal [[synaptic transmission]] is directional and proceeds from the presynaptic to the postsynaptic cell. For induction to occur postsynaptically and be partially expressed presynaptically, a message must travel from the postsynaptic cell to the presynaptic cell in a ''retrograde'' (reverse) direction. Once there, the message presumably initiates a cascade of events that leads to a presynaptic component of expression, such as the increased probability of [[neurotransmitter vesicle]] release.<ref>{{cite journal |author=Zakharenko S, Patterson S, Dragatsis I, Zeitlin S, Siegelbaum S, Kandel E, Morozov A |title=Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses |journal=Neuron |volume=39 |issue=6 |pages=975–90 |year=2003 |pmid=12971897 |doi=10.1016/S0896-6273(03)00543-9}}</ref> |
||
| ⚫ | The |
||
| + | Retrograde signaling is currently a contentious subject as some investigators do not believe the presynaptic cell contributes at all to the expression of LTP.<ref name="riches"/> Even among proponents of the hypothesis there is controversy over the identity of the messenger. Early thoughts focused on [[nitric oxide]], while most recent evidence points to [[cell adhesion]] proteins.<ref name="riches"/> |
||
| ⚫ | The synaptic tag hypothesis may also |
||
| ⚫ | |||
| − | LTP's cooperativity may also be explained by synaptic tagging. While weak stimulation of a single pathway is insufficient to induce LTP, the simultaneous weak stimulation of two pathways is sufficient. As noted previously, weak stimulation initiates the synthesis of a synaptic tag, but is insufficient to trigger late LTP and thus CREB-1-mediated gene expression. But simultaneous weak input converges on kinases that sufficiently activate CREB-1 thereby inducing the synthesis of plasticity-related proteins, which are shipped out cell-wide as described previously. Since a synaptic tag has been synthesized at both synapses, both capture the products of gene expression and both are subsequently potentiated. |
||
| + | Before the local protein synthesis hypothesis gained significant support, there was general agreement that the protein synthesis underlying L-LTP occurred in the cell body. Further, there was thought that the products of this synthesis were shipped cell-wide in a nonspecific manner. It thus became necessary to explain how protein synthesis could occur in the cell body without compromising LTP's input specificity. The synaptic tagging hypothesis attempts to solve the cell's difficult problem of synthesizing proteins in the cell body but ensuring they only reach synapses that have received LTP-inducing stimuli. |
||
| ⚫ | The synaptic tagging hypothesis proposes that a "synaptic tag" is synthesized at synapses that have received LTP-inducing stimuli, and that this synaptic tag may serve to capture plasticity-related proteins shipped cell-wide from the cell body.<ref name="frey-synaptic_tagging">{{cite journal |author=Frey U, Morris R |title=Synaptic tagging and long-term potentiation |journal=Nature |volume=385 |issue=6616 |pages=533–6 |year=1997 |pmid=9020359 |doi=10.1038/385533a0}}</ref> Studies of LTP in the [[Nudibranch|marine snail]] ''[[Aplysia californica]]'' have implicated synaptic tagging as a mechanism for the input-specificity of LTP.<ref>{{cite journal |author=Martin K, Casadio A, Zhu H, Yaping E, Rose J, Chen M, Bailey C, Kandel E |title=Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage |journal=Cell |volume=91 |issue=7 |pages=927–38 |year=1997 |pmid=9428516 |doi=10.1016/S0092-8674(00)80484-5}}</ref><ref>{{cite journal |author=Casadio A, Martin K, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey C, Kandel E |title=A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis |journal=Cell |volume=99 |issue=2 |pages=221–37 |year=1999 |pmid=10535740 |doi=10.1016/S0092-8674(00)81653-0}}</ref> There is some evidence that given two widely separated synapses, an LTP-inducing stimulus at one synapse drives several signaling cascades (described previously) that initiates gene expression in the cell nucleus. At the same synapse (but not the unstimulated synapse), local protein synthesis creates a short-lived (less than three hours) synaptic tag.<!--<ref name="frey-synaptic_tagging" />--> The products of gene expression are shipped globally throughout the cell, but are only captured by synapses that express the synaptic tag. Thus only the synapse receiving LTP-inducing stimuli is potentiated, demonstrating LTP's input specificity. |
||
| − | ==LTP modulation== |
||
| + | |||
| − | {| border="1" style="border-collapse:collapse;float:right;margin-left:10px" cellpadding="3" |
||
| ⚫ | The synaptic tag hypothesis may also account for LTP's associativity and cooperativity. Associativity (''see [[#Properties|Properties]]'') is observed when one synapse is excited with LTP-inducing stimulation while a separate synapse is only weakly stimulated. Whereas one might expect only the strongly stimulated synapse to undergo LTP (since weak stimulation alone is insufficient to induce LTP at either synapse), ''both'' synapses will in fact undergo LTP. While weak stimuli are unable to induce protein synthesis in the cell body, they may prompt the synthesis of a synaptic tag. Simultaneous strong stimulation of a separate pathway, capable of inducing cell body protein synthesis, then may prompt the production of plasticity-related proteins, which are shipped cell-wide. With both synapses expressing the synaptic tag, both would capture the protein products resulting in the expression of LTP in both the strongly stimulated and weakly stimulated pathways. |
||
| − | |+ ''LTP modulators, adapted from (10541462).'' |
||
| + | |||
| ⚫ | |||
| + | Cooperativity is observed when two synapses are activated by weak stimuli incapable of inducing LTP when stimulated individually. But upon simultaneous weak stimulation, both synapses undergo LTP in a cooperative fashion. Synaptic tagging does not explain how multiple weak stimuli can result in a collective stimulus sufficient to induce LTP (this is explained by the postsynaptic summation of EPSPs described previously). Rather, synaptic tagging explains the ability of weakly stimulated synapses, none of which are capable of independently generating LTP, to receive the products of protein synthesis initiated collectively. As before, this may be accomplished through the synthesis of a local synaptic tag following weak synaptic stimulation. |
||
| ⚫ | |||
| + | |||
| + | ===Modulation=== |
||
| + | {| class="wikitable" style="float:right; margin-left:5px" |
||
| ⚫ | |+ Proposed modulators of LTP<ref name="sweatt-kinases">{{cite journal |author=Sweatt J |title=Toward a molecular explanation for long-term potentiation |journal=Learn Mem |volume=6 |issue=5 |pages=399–416 |year=1999 |url=http://www.learnmem.org/cgi/content/full/6/5/399 |pmid=10541462 |doi=10.1101/lm.6.5.399}}</ref> |
||
| ⚫ | |||
| ⚫ | |||
|- |
|- |
||
| − | | [[ |
+ | | [[Adrenergic receptor|β-Adrenergic receptor]] || cAMP, MAPK amplification |
|- |
|- |
||
| ⚫ | |||
| ⚫ | |||
|- |
|- |
||
| − | | [[ |
+ | | [[Dopamine receptor]] || cAMP, MAPK amplification |
|- |
|- |
||
| ⚫ | |||
| ⚫ | |||
|} |
|} |
||
| − | In addition to the signalling pathways described above, hippocampal LTP can be modulated by a variety of molecules. For example, the [[steroid hormone]] [[estradiol]] is one of several molecules that enhances LTP by driving CREB-1 phosphorylation and subsequent [[dendritic spine]] growth (9920677). Additionally, β-adrenergic receptor agonists such as [[norepinephrine]] contribute to the protein synthesis-dependent late phase of LTP (12770561). [[Nitric oxide synthase]] also plays an important role, leading to the up-regulation of nitric oxide and subsequent activation of guanylyl cyclase and PKG, as described previously (10575022). Similarly, activation of [[dopamine receptor]]s enhances LTP via the cAMP/PKA signaling pathway (1833673)(8922403). |
||
| + | As described previously, the molecules that underlie LTP can be classified as mediators or modulators. A mediator of LTP is a molecule, such as the NMDA receptor or calcium, whose presence and activity is necessary for generating LTP under nearly all conditions. By contrast, a modulator is a molecule that can alter LTP but is not essential for its generation or expression.<ref name="riches"/> |
||
| ⚫ | |||
| − | The mere fact that cultured synapses can undergo long-term potentiation when stimulated by electrodes says little about LTP's relation to memory. Several studies have provided some insight as to whether LTP is a requirement for memory. |
||
| + | In addition to the signaling pathways described above, hippocampal LTP may be altered by a variety of modulators. For example, the [[steroid hormone]] [[estradiol]] may enhance LTP by driving CREB phosphorylation and subsequent [[dendritic spine]] growth.<ref>{{cite journal |author=Segal M, Murphy D |title=CREB activation mediates plasticity in cultured hippocampal neurons |journal=Neural Plast |volume=6 |issue=3 |pages=1–7 |year=1999 |pmid=9920677 |doi=10.1155/NP.1998.1}}</ref> Additionally, [[β-adrenergic receptor]] agonists such as [[norepinephrine]] may alter the protein synthesis-dependent late phase of LTP.<ref>{{cite journal |author=Straube T, Frey J |title=Involvement of beta-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength |journal=Neuroscience |volume=119 |issue=2 |pages=473–9 |year=2003 |pmid=12770561 |doi=10.1016/S0306-4522(03)00151-9}}</ref> [[Nitric oxide synthase]] activity may also result in the subsequent activation of guanylyl cyclase and PKG.<ref>{{cite journal |author=Lu Y, Kandel E, Hawkins R |title=Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus |journal=J Neurosci |volume=19 |issue=23 |pages=10250–61 |year=1999 |pmid=10575022}}</ref> Similarly, activation of [[dopamine receptor]]s may enhance LTP through the cAMP/PKA signaling pathway.<ref>{{cite journal |author=Frey U, Matthies H, Reymann K, Matthies H |title=The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro |journal=Neurosci Lett |volume=129 |issue=1 |pages=111–4 |year=1991 |pmid=1833673 |doi=10.1016/0304-3940(91)90732-9}}</ref><ref>{{cite journal |author=Otmakhova N, Lisman J |title=D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses |journal=J Neurosci |volume=16 |issue=23 |pages=7478–86 |year=1996 |pmid=8922403}}</ref> |
||
| − | ===NMDA blockade=== |
||
| − | Richard Morris provided some of the first evidence that LTP was indeed required for the formation of memories [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2869411]. He tested the [[spatial memory]] of two groups of rats, one whose hippocampi were bathed in the NMDA receptor blocker [[APV]], and the other acting as a control group. (The hippocampus, where LTP was originally observed, is required for some forms of spatial learning). Both groups were then subjected to the [[Morris water maze]], in which rats were placed into a circular pool of murky water (often made opaque with milk powder or white paint) and tested on how quickly they could locate a platform hidden just beneath the water's surface. |
||
| ⚫ | |||
| − | Rats in the control group were able to locate the platform and escape from the pool, whereas the ability of APV-treated rats to complete the task was significantly impaired. Moreover, when slices of the [[hippocampus]] were taken from both groups of rats, LTP was easily induced in controls, but could not be induced in the brains of APV-treated rats. This provided some evidence that the NMDA receptor — and thus LTP — was somehow involved with at least some types of learning and memory. |
||
| + | While the long-term potentiation of synapses in cell culture seems to provide an elegant substrate for learning and memory, the contribution of LTP to behavioral learning — that is, learning at the level of the whole organism — cannot simply be extrapolated from ''in vitro'' studies. For this reason, considerable effort has been dedicated to establishing whether LTP is a requirement for learning and memory in living animals. |
||
| + | ===Spatial memory=== |
||
| − | Similarly, [[Susumu Tonegawa]] has demonstrated that a specific region of the hippocampus, namely CA1, is crucial to the formation of spatial memories [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8980239]. So-called ''[[place cell]]s'' located in this region fire when the rat is in a particular location in the environment. Since a large group of these cells will have place fields evenly distributed throughout the environment, one interpretation is that these cells form a sort of map. The accuracy of these maps determines how well a rat learns about its environment, and thus how well it can navigate about it. |
||
| + | [[Image:MorrisWaterMaze.jpg|thumb|250px|The [[Morris water maze]] task has been used to demonstrate the necessity of NMDA receptors in establishing [[spatial memory|spatial memories]].]] |
||
| + | In 1986, Richard Morris provided some of the first evidence that LTP was indeed required for the formation of memories ''in vivo''.<ref>{{cite journal |author=Morris R, Anderson E, Lynch G, Baudry M |title=Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5 |journal=Nature |volume=319 |issue=6056 |pages=774–6 |year=1986 |pmid=2869411 |doi=10.1038/319774a0}}</ref> He tested the [[spatial memory]] of rats by pharmacologically modifying their hippocampus, a brain structure whose role in spatial learning is well established. Rats were trained on the [[Morris water maze]], a spatial memory task in which rats swim in a pool of murky water until they locate the platform hidden beneath its surface. During this exercise, normal rats are expected to associate the location of the hidden platform with salient cues placed at specific positions around the circumference of the maze. After training, one group of rats had their hippocampi bathed in the NMDA receptor blocker [[APV (NMDAR antagonist)|APV]], while the other group served as the [[control group|control]]. Both groups were then subjected to the water maze spatial memory task. Rats in the control group were able to locate the platform and escape from the pool, while the performance of APV-treated rats was significantly impaired. Moreover, when slices of the hippocampus were taken from both groups, LTP was easily induced in controls, but could not be induced in the brains of APV-treated rats. This provided early evidence that the NMDA receptor — and by extension, LTP — was required for at least some types of learning and memory. |
||
| − | Tonegawa found that by impairing the NMDA receptor, specifically by [[genetics|genetically]] removing the NMDAR1 subunit in the CA1 region, the place fields generated were substantially less specific than those of controls. That is, rats produced faulty spatial maps when their NMDA receptors were impaired. As expected, these rats performed very poorly on spatial tasks compared to controls, providing more support to the notion that LTP is the underlying mechanism of spatial learning. |
||
| + | Similarly, [[Susumu Tonegawa]] demonstrated in 1996 that the CA1 area of the hippocampus is crucial to the formation of spatial memories in living mice.<ref>{{cite journal |author=McHugh T, Blum K, Tsien J, Tonegawa S, Wilson M |title=Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice |journal=Cell |volume=87 |issue=7 |pages=1339–49 |year=1996 |pmid=8980239 |doi=10.1016/S0092-8674(00)81828-0}}</ref> So-called ''[[place cell]]s'' located in this region become active only when the rat is in a particular location — called a ''place field'' — in the environment. Since these place fields are distributed throughout the environment, one interpretation is that groups of place cells form maps in the hippocampus. The accuracy of these maps determines how well a rat learns about its environment and thus how well it can navigate it. Tonegawa found that by impairing the NMDA receptor, specifically by genetically removing the NR1 subunit in the CA1 region, the place fields generated were substantially less specific than those of controls. That is, rats produced faulty spatial maps when their NMDA receptors were impaired. As expected, these rats performed very poorly on spatial tasks compared to controls, further supporting the role of LTP in spatial learning. |
||
| − | ===Doogie mice=== |
||
| − | Enhanced NMDA receptor activity in the hippocampus has also been shown to produce enhanced LTP and an overall improvement in spatial learning. [http://www.bumc.bu.edu/www/busm/pharmacology/tsien/index.htm Joe Tsien] produced a line of [[mus musculus|mice]] with enhanced NMDA receptor function by overexpressing the NR2B subunit in the hippocampus [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11640933]. These smart mice, nicknamed "Doogie mice" after the prodigious doctor [[Doogie Howser]], had larger long-term potentiation and excelled at spatial learning tasks, once again suggesting LTP's involvement in the formation of hippocampal-dependent memories [http://www.abc.net.au/quantum/stories/s103200.htm]. |
||
| + | Enhanced NMDA receptor activity in the hippocampus has also been shown to produce enhanced LTP and an overall improvement in spatial learning. In 2001, Joe Tsien produced a line of mice with enhanced NMDA receptor function by overexpressing the NR2B subunit in the hippocampus.<ref name="pmid10485705">{{cite journal | author = Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ | title = Genetic enhancement of learning and memory in mice | journal = Nature | volume = 401 | pages = 63–69 | year = 1999 | pmid = 10485705 | url = http://www.nature.com/nature/journal/v401/n6748/abs/401063a0.html | doi = 10.1038/43432 }}</ref><ref>{{cite journal |author=Tang Y, Wang H, Feng R, Kyin M, Tsien J |title=Differential effects of enrichment on learning and memory function in NR2B transgenic mice |journal=Neuropharmacology |volume=41 |issue=6 |pages=779–90 |year=2001 |pmid=11640933 |doi=10.1016/S0028-3908(01)00122-8}}</ref> The resulting smart mice, nicknamed "Doogie mice" after the fictional prodigious doctor [[Doogie Howser]], had larger LTP and excelled at spatial learning tasks, reinforcing LTP's importance in the formation of hippocampus-dependent memories. |
||
| ⚫ | |||
| − | * [[learning]] |
||
| ⚫ | |||
| ⚫ | |||
| + | ===Inhibitory avoidance=== |
||
| − | ==Notes== |
||
| + | In 2006, Jonathan Whitlock and colleagues reported on a series of experiments that provided perhaps the strongest evidence of LTP's role in behavioral memory, arguing that to conclude that LTP underlies behavioral learning, the two processes must both mimic and occlude one another.<ref>{{cite journal |author=Whitlock J, Heynen A, Shuler M, Bear M |title=Learning induces long-term potentiation in the hippocampus |journal=Science |volume=313 |issue=5790 |pages=1093–7 |year=2006 |pmid=16931756 |doi=10.1126/science.1128134}}</ref> Employing an inhibitory avoidance learning paradigm, researchers trained rats in a two-chambered apparatus with light and dark chambers, the latter being fitted with a device that delivered a foot shock to the rat upon entry. An analysis of CA1 hippocampal synapses revealed that inhibitory avoidance training induced ''in vivo'' AMPA receptor phosphorylation of the same type as that seen in LTP ''in vitro''; that is, inhibitory avoidance training mimicked LTP. In addition, synapses potentiated during training could not be further potentiated by experimental manipulations that would have otherwise induced LTP; that is, inhibitory avoidance training occluded LTP. In a response to the article, Timothy Bliss and colleagues remarked that these and related experiments "substantially advance the case for LTP as a neural mechanism for memory."<ref>{{cite journal |author=Bliss T, Collingridge G, Laroche S |title=Neuroscience. ZAP and ZIP, a story to forget |journal=Science |volume=313 |issue=5790 |pages=1058–9 |year=2006 |pmid=16931746 |doi=10.1126/science.1132538}}</ref> |
||
| − | #{{note|hebb-org}}{{cite book | author=Hebb, D. O. | title=Organization of Behavior: a Neuropsychological Theory | publisher=New York: John Wiley | year=1949 | id=ISBN 0471367273}} |
||
| + | |||
| − | #{{note|lomo-discovery}}{{cite journal | author=Terje Lømo | title=The discovery of long-term potentiation | journal=Philos Trans R Soc Lond B Biol Sci | volume=358 | issue=1432 | year=2003 | pages=617-20 | id=PMID 12740104}} |
||
| + | ==Clinical significance== |
||
| − | #{{note|blisslomo}}{{cite journal | author=Bliss TV, Lomo T | title=Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path | journal=J Physiol | year=1973 | pages=331-56 | volume=232 | issue=2 | id=PMID 4727084}} |
||
| + | The role of LTP in disease is less clear than its role in basic mechanisms of [[synaptic plasticity]]. However, alterations in LTP may contribute to a number of [[neurological disease]]s, including [[Major depressive disorder|depression]], [[Parkinson's disease]], [[epilepsy]], and [[neuropathic pain]].<ref name="cooke-clinical">{{cite journal |author=Cooke SF, Bliss TV |title=Plasticity in the human central nervous system |journal=Brain: A Journal of Neurology |volume=129 |issue=Pt 7 |pages=1659–73 |year=2006 |month=July |pmid=16672292 |doi=10.1093/brain/awl082 |url=http://brain.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=16672292}}</ref> Impaired LTP may also have a role in [[Alzheimer's disease]] and [[drug addiction]]. |
||
| − | #{{note|mayer-nmda_mgblock}}{{cite journal | author=Mayer ML, Westbrook GL, Guthrie PB | title=Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones | journal=Nature | volume=309 | issue=5965 | year=1984 | pages=261-3 | id=PMID 6325946}} |
||
| + | |||
| − | #{{note|rogan-fearcond}}{{cite journal | author=Rogan MT, Staubli UV, LeDoux JE | title=Fear conditioning induces associative long-term potentiation in the amygdala | journal=Nature | volume=390 | issue=6660 | year=1997 | pages=604-7 | id=PMID 9403688}} |
||
| + | ===Alzheimer's disease=== |
||
| − | #{{note|artola-visualcortex}}{{cite journal | author=Artola A, Singer W | title=Long-term potentiation and NMDA receptors in rat visual cortex | journal=Nature | volume=330 | issue=6149 | year=1987 | pages=649-52 | id=PMID 2446147}} |
||
| + | [[Image:APP and LTP in Alzheimer disease.png|thumb|150px|Misprocessing of [[amyloid precursor protein]] (APP) in [[Alzheimer's disease]] disrupts LTP and is thought to lead to early cognitive decline in individuals with the disease.<ref name="pmid12740129"/>]] |
||
| − | #{{note|weisskopf-ca_mediated_ltp}}{{cite journal | author=Weisskopf MG, Bauer EP, LeDoux JE | title=L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala | journal=J Neurosci | volume=19 | issue=23 | year=1999 | pages=10512-9 | id=PMID 10575047}} |
||
| + | |||
| − | #{{note|lu-nitricoxide}}{{cite journal | author=Lu YF, Kandel ER, Hawkins RD | title=Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus | journal=J Neurosci | volume=19 | issue=23 | year=1999 | pages=10250-61 | id=PMID 10575022}} |
||
| + | LTP has received much attention among those who study [[Alzheimer's disease]] (AD), a [[neurodegenerative disease]] that causes marked cognitive decline and [[dementia]]. Much of this deterioration occurs in association with degenerative changes in the hippocampus and other [[medial temporal lobe]] structures. Because of the hippocampus' well established role in LTP, some have suggested that the cognitive decline seen in individuals with AD may result from impaired LTP. |
||
| − | #{{note|kandel-tetanization}}{{cite journal | author=Huang YY, Kandel ER | title=Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. | journal=Learn Mem | year=1994 | pages=74-82 | volume=1 | issue=1 | id=PMID 10467587}} |
||
| + | |||
| − | #{{note|kojima-fyn}}{{cite journal | author=Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER | title=Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene | journal=Proc Natl Acad Sci U S A | volume=94 | issue=9 | year=1997 | pages=4761-5 | id=PMID 9114065}} |
||
| + | In a 2003 review of the literature, Rowan ''et al.'' proposed one model for how LTP might be affected in AD.<ref name="pmid12740129">{{cite journal |author=Rowan MJ, Klyubin I, Cullen WK, Anwyl R |title=Synaptic plasticity in animal models of early Alzheimer's disease |journal=Philosophical transactions of the Royal Society of London. Series B, Biological sciences |volume=358 |issue=1432 |pages=821–8 |year=2003 |month=April |pmid=12740129 |pmc=1693153 |doi=10.1098/rstb.2002.1240 |url=http://journals.royalsociety.org/openurl.asp?genre=article&issn=0962-8436&volume=358&issue=1432&spage=821}}</ref> AD appears to result, at least in part, from misprocessing of [[amyloid precursor protein]] (APP). The result of this abnormal processing is the accumulation of fragments of this protein, called [[amyloid β]] (Aβ). Aβ exists in both soluble and fibrillar forms. Misprocessing of APP results in the accumulation of soluble Aβ that, according to Rowan's hypothesis, impairs hippocampal LTP and may lead to the cognitive decline seen early in AD. |
||
| − | #{{note|malinow-pkc_camkii_inhib}}{{cite journal | author=Malinow R, Schulman H, Tsien RW | title=Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP | journal=Science | volume=245 | issue=4920 | year=1989 | pages=862-6 | id=PMID 2549638}} |
||
| + | |||
| − | #{{note|otmakhova-camp_inhibition}}{{cite journal | author=Otmakhova NA, Otmakhov N, Mortenson LH, Lisman JE | title=Inhibition of the cAMP pathway decreases early long-term potentiation at CA1 hippocampal synapses | journal=J Neurosci | volume=20 | issue=12 | year=2000 | pages=4446-51 | id=PMID 10844013}} |
||
| + | AD may also impair LTP through mechanisms distinct from Aβ. For example, one study demonstrated that the enzyme PKMζ accumulates in [[neurofibrillary tangle]]s, which are a pathologic marker of AD. PKMζ is an enzyme with critical importance in the [[#Maintenance_2|maintenance of late LTP]].<ref name="pmid16691113">{{cite journal |author=Crary JF, Shao CY, Mirra SS, Hernandez AI, Sacktor TC |title=Atypical protein kinase C in neurodegenerative disease I: PKMzeta aggregates with limbic neurofibrillary tangles and AMPA receptors in Alzheimer disease |journal=Journal of neuropathology and experimental neurology |volume=65 |issue=4 |pages=319–26 |year=2006 |month=April |pmid=16691113 |doi=10.1097/01.jnen.0000218442.07664.04 |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?an=00005072-200604000-00002}}</ref> |
||
| − | #{{note|english-mapk_required}}{{cite journal | author=English JD, Sweatt JD | title=A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation | journal=J Biol Chem | volume=272 | issue=31 | year=1997 | pages=19103-6 | id=PMID 9235897}} |
||
| + | |||
| − | #{{note|sweatt-mapk}}{{cite journal | author=Sweatt JD | title=The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory | journal=J Neurochem | volume=76 | issue=1 | year=2001 | pages=1-10 | id=PMID 11145972}} |
||
| + | ===Drug addiction=== |
||
| − | #{{note|huang-tyrosinekinase}}{{cite journal | author=Huang CC, Hsu KS | title=Protein tyrosine kinase is required for the induction of long-term potentiation | journal=J Physiol | year=1999 | volume=520 Pt 3 | pages=783-96 | id=PMID 10545144}} |
||
| + | Research in the field of [[addiction medicine]] has also recently turned its focus to LTP, owing to the hypothesis that [[drug addiction]] represents a powerful form of learning and memory.<ref name="pmid17948030">{{cite journal |author=Kauer JA, Malenka RC |title=Synaptic plasticity and addiction |journal=Nature reviews. Neuroscience |volume=8 |issue=11 |pages=844–58 |year=2007 |month=November |pmid=17948030 |doi=10.1038/nrn2234}}</ref> Addiction is a complex neurobehavioral phenomenon involving various parts of the brain, such as the [[ventral tegmental area]] (VTA) and [[nucleus accumbens]] (NAc). Studies have demonstrated that VTA and NAc synapses are capable of undergoing LTP<ref name="pmid17948030"/> and that this LTP may be responsible for the behaviors that characterize addiction.<ref name="pmid14993438">{{cite journal |author=Wolf ME |title=LTP may trigger addiction |journal=Molecular interventions |volume=3 |issue=5 |pages=248–52 |year=2003 |month=August |pmid=14993438 |doi=10.1124/mi.3.5.248 |url=http://molinterv.aspetjournals.org/cgi/pmidlookup?view=long&pmid=14993438}}</ref> |
||
| − | #{{note|malenka-calmodulin_pka}}{{cite journal | author=Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN | title=An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. | journal=Nature | year=1989 | pages=554-7 | volume=340 | issue=6234 | id=PMID 2549423}} |
||
| + | |||
| ⚫ | |||
| ⚫ | |||
| − | #{{note|esteban-ampa_trafficking}}{{cite journal | author=Esteban JA | title=AMPA receptor trafficking: a road map for synaptic plasticity. | journal=Mol Interv | year=2003 | pages=375-85 | volume=3 | issue=7 | id=PMID 14993459}} |
||
| + | * [[Synaptic plasticity]] |
||
| − | #{{note|nguyen-transcription}}{{cite journal | author=Nguyen PV, Abel T, Kandel ER | title=Requirement of a critical period of transcription for induction of a late phase of LTP. | journal=Science | year=1994 | pages=1104-7 | volume=265 | issue=5175 | id=PMID 8066450}} |
||
| + | * [[Neuroplasticity]] |
||
| − | #{{note|bourtchuladze-camp-mut}}{{cite journal | author=Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ | title=Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. | journal=Cell | year=1994 | pages=59-68 | volume=79 | issue=1 | id=PMID 7923378}} |
||
| ⚫ | |||
| − | #{{note|frey-translation}}{{cite journal | author=Frey U, Krug M, Reymann KG, Matthies H | title=Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. | journal=Brain Res | year=1988 | pages=57-65 | volume=452 | issue=1-2 | id=PMID 3401749}} |
||
| ⚫ | |||
| − | #{{note|poser-ac}}{{cite journal | author=Poser S, Storm DR | title=Role of Ca2+-stimulated adenylyl cyclases in LTP and memory formation. | journal=Int J Dev Neurosci | year=2001 | pages=387-94 | volume=19 | issue=4 | id=PMID 11378299}} |
||
| − | #{{note|dash-cre-inj}}{{cite journal | author=Dash PK, Hochner B, Kandel ER | title=Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. | journal=Nature | year=1990 | pages=718-21 | volume=345 | issue=6277 | id=PMID 2141668}} |
||
| − | #{{note|bartsch-creb}}{{cite journal | author=Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER | title=CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. | journal=Cell | year=1998 | pages=211-23 | volume=95 | issue=2 | id=PMID 9790528}} |
||
| − | #{{note|kasahara-pkiv}}{{cite journal | author=Kasahara J, Fukunaga K, Miyamoto E | title=Activation of calcium/calmodulin-dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. | journal=J Biol Chem | year=2001 | pages=24044-50 | volume=276 | issue=26 | id=PMID 11306573}} |
||
| − | #{{note|huang-pka-mapk}}{{cite journal | author=Huang YY, Martin KC, Kandel ER | title=Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. | journal=J Neurosci | year=2000 | pages=6317-25 | volume=20 | issue=17 | id=PMID 10964936}} |
||
| − | #{{note|impey-erk-pka}}{{cite journal | author=Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR | title=Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. | journal=Neuron | year=1998 | pages=869-83 | volume=21 | issue=4 | id=PMID 9808472}} |
||
| − | #{{note|segal-creb}}{{cite journal | author=Segal M, Murphy DD | title=CREB activation mediates plasticity in cultured hippocampal neurons. | journal=Neural Plast | year=1998 | pages=1-7 | volume=6 | issue=3 | id=PMID 9920677}} |
||
| − | #{{note|lu-no}}{{cite journal | author=Lu YF, Kandel ER, Hawkins RD | title=Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. | journal=J Neurosci | year=1999 | pages=10250-61 | volume=19 | issue=23 | id=PMID 10575022}} |
||
| − | #{{note|alkadhi-retro_co}}{{cite journal | author=Alkadhi KA, Al-Hijailan RS, Malik K, Hogan YH | title=Retrograde carbon monoxide is required for induction of long-term potentiation in rat superior cervical ganglion. | journal=J Neurosci | year=2001 | pages=3515-20 | volume=21 | issue=10 | id=PMID 11331380}} |
||
| − | #{{note|kato-retro_paf}}{{cite journal | author=Kato K, Zorumski CF | title=Platelet-activating factor as a potential retrograde messenger. | journal=J Lipid Mediat Cell Signal | year=1996 | pages=341-8 | volume=14 | issue=1-3 | id=PMID 8906580}} |
||
| − | #{{note|kato-retro_paf2}}{{cite journal | author=Kato K, Clark GD, Bazan NG, Zorumski CF | title=Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. | journal=Nature | year=1994 | pages=175-9 | volume=367 | issue=6459 | id=PMID 8114914}} |
||
| − | #{{note|arancio-retro_no}}{{cite journal | author=Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD | title=Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. | journal=Cell | year=1996 | pages=1025-35 | volume=87 | issue=6 | id=PMID 8978607}} |
||
| − | #{{note|hawkins-retro_no}}{{cite journal | author=Hawkins RD, Son H, Arancio O | title=Nitric oxide as a retrograde messenger during long-term potentiation in hippocampus. | journal=Prog Brain Res | year=1998 | pages=155-72 | volume=118 | id=PMID 9932440}} |
||
| − | #{{note|frey-synaptic_tagging}}{{cite journal | author=Frey U, Morris RG | title=Synaptic tagging and long-term potentiation. | journal=Nature | year=1997 | pages=533-6 | volume=385 | issue=6616 | id=PMID 9020359}} |
||
| − | #{{note|martin-synaptic_tagging_aplysia}}{{cite journal | author=Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER | title=Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. | journal=Cell | year=1997 | pages=927-38 | volume=91 | issue=7 | id=PMID 9428516}} |
||
| − | #{{note|casadio-local_protein_synthesis}}{{cite journal | author=Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER | title=A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. | journal=Cell | year=1999 | pages=221-37 | volume=99 | issue=2 | id=PMID 10535740}} |
||
==References== |
==References== |
||
| + | <div class="references-small" style="-moz-column-count:2; column-count:2;"> |
||
| − | * Deadwyler SA, Dunwiddie T, Lynch G. "A critical level of protein synthesis is required for long-term potentiation." ''Synapse''. 1987;1(1):90-5. PMID 3505366 |
||
| + | <references /> |
||
| − | * Frey U, Morris RG. "Synaptic tagging and long-term potentiation." ''Nature''. 1997 Feb 6;385(6616):533-6. PMID 9020359 |
||
| + | </div> |
||
| − | * Bennett MR. "The concept of long term potentiation of transmission at synapses." ''Prog Neurobiol.'' 2000 Feb;60(2):109-37. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10639051&dopt=Abstract PMID 10639051] |
||
| + | |||
| − | * Collingridge GL, Kehl SJ, McLennan H. "Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus." ''J Physiol.'' 1983 Jan;334:33-46. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6306230&dopt=Abstract PMID 6306230] |
||
| + | ==Further reading== |
||
| − | * Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 2004 Sep 30;44(1):5-21. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15450156&query_hl=4] |
||
| + | *{{cite book |author=Bliss, T; Collingridge, G; Morris, R |title=Long-term potentiation: enhancing neuroscience for 30 years |publisher=Oxford University Press |location=Oxford |year=2004 |pages= |isbn=0-19-853030-7 |oclc= |doi= |accessdate=}} |
||
| − | * Martin SJ, Grimwood PD, Morris RG. "Synaptic plasticity and memory: an evaluation of the hypothesis." ''Annu Rev Neurosci.'' 2000;23:649-711. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10845078 PMID 10845078] |
||
| − | * McNaughton BL, Douglas RM, Goddard GV. "Synaptic enhancement in fascia dentata: cooperativity among coactive afferents." ''Brain Res.'' 1978 Nov 24;157(2):277-93. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=719524&dopt=Abstract PMID 719524] |
||
| − | * Murphy GG, Glanzman DL. "Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+." ''Proc Natl Acad Sci U S A''. 1996 Sep 3;93(18):9931-6. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8790434&dopt=Abstract PMID 8790434] ([http://www.pnas.org/cgi/reprint/93/18/9931.pdf full text PDF]) |
||
| − | * Morris RG, Anderson E, Lynch GS, Baudry M. "Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5." ''Nature''. 1986 Feb 27-Mar 5;319(6056):774-6. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=2869411 PMID 2869411] |
||
| − | * McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. "Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice." ''Cell''. 1996 Dec 27;87(7):1147-8. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8980239 PMID 8980239] |
||
| − | * Pavlidis, P., Mongomery, J. and Madison, D.V. (2000). Presynaptic Protein Kinase Activity Supports Long-Term Potentiation at Synapses Between Individual Hippocampal Neurons. Journal of Neuroscience 20, 4497 4505. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10844019&query_hl=6] |
||
| − | * Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. "Differential effects of enrichment on learning and memory function in NR2B transgenic mice." ''Neuropharmacology''. 2001 Nov;41(6):779-90. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11640933 PMID 11640933] |
||
| − | * Hebb, D.O. (1949) The organization of behavior. Wiley, New York. |
||
==External links== |
==External links== |
||
| + | * [http://www.physorg.com/news75650360.html Researchers provide first evidence for learning mechanism], a [[PhysOrg.com]] report on 2006 study by Bear and colleagues. |
||
| − | * [http://www.sciam.com/article.cfm?articleID=00075ED9-33D5-1C75-9B81809EC588EF21&pageNumber=1&catID=4 Scientific American article of the doogie mice.] |
||
| − | + | *[http://news.bbc.co.uk/olmedia/435000/video/_435883_pallab9_vi.ram Short video documentary about the Doogie mice.] ([[RealPlayer]] format) |
|
| + | * [http://www.abc.net.au/quantum/stories/s103200.htm "Smart Mouse", a Quantum ABC TV episode about the Doogie mice.] |
||
| + | * {{MeshName|Long-Term+Potentiation}} |
||
| + | |||
| + | {{Nervous system physiology}} |
||
| + | {{DEFAULTSORT:Long-Term Potentiation}} |
||
[[Category:Neurophysiology]] |
[[Category:Neurophysiology]] |
||
| − | [[Category: |
+ | [[Category:Memory]] |
| + | [[Category:Behavioral neuroscience]] |
||
| + | <!-- |
||
[[de:Langzeit-Potenzierung]] |
[[de:Langzeit-Potenzierung]] |
||
| + | [[es:Potenciación a largo plazo]] |
||
[[is:Langtímaefling]] |
[[is:Langtímaefling]] |
||
| + | [[it:Long term potentiation]] |
||
| + | [[nl:Langetermijnpotentiatie]] |
||
| + | [[ja:長期増強]] |
||
| + | [[pl:Długotrwałe wzmocnienie synaptyczne]] |
||
| + | [[pt:Potenciação de longa duração]] |
||
| + | [[ru:Долговременная потенциация]] |
||
[[sv:Long-term potentiation]] |
[[sv:Long-term potentiation]] |
||
| + | [[uk:Довготривала потенціація]] |
||
| + | --> |
||
{{enWP|Long-term potentiation}} |
{{enWP|Long-term potentiation}} |
||
Latest revision as of 05:47, 31 December 2009
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Cognitive Psychology: Attention · Decision making · Learning · Judgement · Memory · Motivation · Perception · Reasoning · Thinking - Cognitive processes Cognition - Outline Index
- Main article: Postactivation potentials
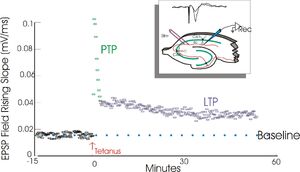
Long-term potentiation (LTP) is a persistent increase in synaptic strength following high-frequency stimulation of a chemical synapse. Studies of LTP are often carried out in slices of the hippocampus, an important organ for learning and memory. In such studies, electrical recordings are made from cells and plotted in a graph such as this one. This graph compares the response to stimuli in synapses that have undergone LTP versus synapses that have not undergone LTP. Synapses that have undergone LTP tend to have stronger electrical responses to stimuli than other synapses. The term long-term potentiation comes from the fact that this increase in synaptic strength, or potentiation, lasts a very long time compared to other processes that affect synaptic strength.[1]
In neuroscience, long-term potentiation (LTP) is a long-lasting enhancement in signal transmission between two neurons that results from stimulating them synchronously.[2] It is one of several phenomena underlying synaptic plasticity, the ability of chemical synapses to change their strength. As memories are thought to be encoded by modification of synaptic strength,[3] LTP is widely considered one of the major cellular mechanisms that underlies learning and memory.[2][3]
LTP shares many features with long-term memory, making it an attractive candidate for a cellular mechanism of learning. For example, LTP and long-term memory are triggered rapidly, each depends upon the synthesis of new proteins, each has properties of associativity, and each can last for many months.[2] LTP may account for many types of learning, from the relatively simple classical conditioning present in all animals, to the more complex, higher-level cognition observed in humans.[2]
At a cellular level, LTP enhances synaptic transmission. It improves the ability of two neurons, one presynaptic and the other postsynaptic, to communicate with one another across a synapse. The precise molecular mechanisms for this enhancement of transmission have not been fully established, in part because LTP is governed by multiple mechanisms that vary by species and brain region. In the most well understood form of LTP, enhanced communication is predominantly carried out by improving the postsynaptic cell's sensitivity to signals received from the presynaptic cell.[4] These signals, in the form of neurotransmitter molecules, are received by neurotransmitter receptors present on the surface of the postsynaptic cell. LTP improves the postsynaptic cell's sensitivity to neurotransmitter in large part by increasing the activity of existing receptors and by increasing the number of receptors on the postsynaptic cell surface.[4]
LTP was discovered in the rabbit hippocampus by Terje Lømo in 1966 and has remained a popular subject of research since. Many modern LTP studies seek to better understand its basic biology, while others aim to draw a causal link between LTP and behavioral learning. Still others try to develop methods, pharmacologic or otherwise, of enhancing LTP to improve learning and memory. LTP is also a subject of clinical research, for example, in the areas of Alzheimer's disease and addiction medicine.
History
Early theories of learning

The 19th century neuroanatomist Santiago Ramón y Cajal proposed that memories might be stored across synapses, the junctions between neurons that allow for their communication.
At the end of the 19th century, scientists generally recognized that the number of neurons in the adult brain (roughly 100 billion[5]) did not increase significantly with age, giving neurobiologists good reason to believe that memories were generally not the result of new neuron production.[6] With this realization came the need to explain how memories could form in the absence of new neurons.
The Spanish neuroanatomist Santiago Ramón y Cajal was among the first to suggest a mechanism of learning that did not require the formation of new neurons. In his 1894 Croonian Lecture, he proposed that memories might instead be formed by strengthening the connections between existing neurons to improve the effectiveness of their communication.[6] Hebbian theory, introduced by Donald Hebb in 1949, echoed Ramón y Cajal's ideas, further proposing that cells may grow new connections or undergo metabolic changes that enhance their ability to communicate:
Let us assume that the persistence or repetition of a reverberatory activity (or "trace") tends to induce lasting cellular changes that add to its stability.... When an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A's efficiency, as one of the cells firing B, is increased.[7]
Though these theories of memory formation are now well established, they were farsighted for their time: late 19th and early 20th century neuroscientists and psychologists were not equipped with the neurophysiological techniques necessary for elucidating the biological underpinnings of learning in animals. These skills would not come until the latter half of the 20th century, at about the same time as the discovery of long-term potentiation.
Discovery
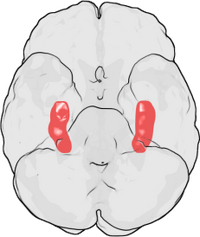
LTP was first discovered in the rabbit hippocampus. In humans, the hippocampus is located in the medial temporal lobe. This illustration of the underside of the human brain shows the hippocampus highlighted in red. The frontal lobe is at the top of the illustration and the occipital lobe is at the bottom.
LTP was first observed by Terje Lømo in 1966 in the Oslo, Norway, laboratory of Per Andersen.[8][9] There, Lømo conducted a series of neurophysiological experiments on anesthetized rabbits to explore the role of the hippocampus in short-term memory.
Lømo's experiments focused on connections, or synapses, from the perforant pathway to the dentate gyrus. These experiments were carried out by stimulating presynaptic fibers of the perforant pathway and recording responses from a collection of postsynaptic cells of the dentate gyrus. As expected, a single pulse of electrical stimulation to fibers of the perforant pathway caused excitatory postsynaptic potentials (EPSPs) in cells of the dentate gyrus. What Lømo unexpectedly observed was that the postsynaptic cells' response to these single-pulse stimuli could be enhanced for a long period of time if he first delivered a high-frequency train of stimuli to the presynaptic fibers. When such a train of stimuli was applied, subsequent single-pulse stimuli elicited stronger, prolonged EPSPs in the postsynaptic cell population. This phenomenon, whereby a high-frequency stimulus could produce a long-lived enhancement in the postsynaptic cells' response to subsequent single-pulse stimuli, was initially called "long-lasting potentiation".[10][11]
Timothy Bliss, who joined the Andersen laboratory in 1968,[8] collaborated with Lømo and in 1973 the two published the first characterization of long-lasting potentiation in the rabbit hippocampus.[10] Bliss and Tony Gardner-Medwin published a similar report of long-lasting potentiation in the awake animal which appeared in the same issue as the Bliss and Lømo report.[11] In 1975, Douglas and Goddard proposed "long-term potentiation" as a new name for the phenomenon of long-lasting potentiation.[12][13] Andersen suggested that the authors chose "long-term potentiation" perhaps because of its easily pronounced acronym, "LTP".[14]
Types
Since its original discovery in the rabbit hippocampus, LTP has been observed in a variety of other neural structures, including the cerebral cortex, cerebellum, amygdala,[15] and many others. Robert Malenka, a prominent LTP researcher, has suggested that LTP may even occur at all excitatory synapses in the mammalian brain.[4]
Different areas of the brain exhibit different forms of LTP. The specific type of LTP exhibited between neurons depends on a number of factors. One such factor is the age of the organism when LTP is observed. For example, the molecular mechanisms of LTP in the immature hippocampus differ from those mechanisms that underlie LTP of the adult hippocampus.[16] The signalling pathways used by a particular cell also contribute to the specific type of LTP present. For example, some types of hippocampal LTP depend on the NMDA receptor, others may depend upon the metabotropic glutamate receptor (mGluR), while still others depend upon another molecule altogether.[4] The variety of signaling pathways that contribute to LTP and the wide distribution of these various pathways in the brain are reasons that the type of LTP exhibited between neurons depends in part upon the anatomic location in which LTP is observed. For example, LTP in the Schaffer collateral pathway of the hippocampus is NMDA receptor-dependent, whereas LTP in the mossy fiber pathway is NMDA receptor-independent.[17]
The pre- and postsynaptic activity required to induce LTP are other criteria by which LTP is classified. Broadly, this allows classification of LTP into Hebbian, non-Hebbian, and anti-Hebbian mechanisms. Borrowing its name from Hebb's postulate, summarized by the maxim that "cells that fire together wire together," Hebbian LTP requires simultaneous pre- and postsynaptic depolarization for its induction.[18] Non-Hebbian LTP is a type of LTP that does not require such simultaneous depolarization of pre- and postsynaptic cells; an example of this occurs in the mossy fiber hippocampal pathway.[19] A special case of non-Hebbian LTP, anti-Hebbian LTP explicitly requires simultaneous presynaptic depolarization and relative postsynaptic hyperpolarization for its induction.[20]
Owing to its predictable organization and readily inducible LTP, the CA1 hippocampus has become the prototypical site of mammalian LTP study. In particular, NMDA receptor-dependent LTP in the adult CA1 hippocampus is the most widely studied type of LTP,[4] and is therefore the focus of this article.
Properties
NMDA receptor-dependent LTP exhibits several properties, including input specificity, associativity, cooperativity, and persistence.
- Input specificity
- Once induced, LTP at one synapse does not spread to other synapses; rather LTP is input specific. Long-term potentiation is only propagated to those synapses according to the rules of associativity and cooperativity. However, the input specificity of LTP may be incomplete at short distances.
- Associativity
- Associativity refers to the observation that when weak stimulation of a single pathway is insufficient for the induction of LTP, simultaneous strong stimulation of another pathway will induce LTP at both pathways.
- Cooperativity
- LTP can be induced either by strong tetanic stimulation of a single pathway to a synapse, or cooperatively via the weaker stimulation of many. When one pathway into a synapse is stimulated weakly, it produces insufficient postsynaptic depolarization to induce LTP. In contrast, when weak stimuli are applied to many pathways that converge on a single patch of postsynaptic membrane, the individual postsynaptic depolarizations generated may collectively depolarize the postsynaptic cell enough to induce LTP cooperatively. Synaptic tagging, discussed later, may be a common mechanism underlying associativity and cooperativity. Bruce McNaughton argues that any difference between associativity and cooperativity is strictly semantic.[21]
- Persistence
- LTP is persistent, lasting from several minutes to many months, and it is this persistence that separates LTP from other forms of synaptic plasticity.[22]
Mechanism
Long-term potentiation occurs through a variety of mechanisms throughout the nervous system; no single mechanism unites all of LTP's many types. However, for the purposes of study, LTP is commonly divided into three phases that occur sequentially: short-term potentiation, early LTP, and late LTP.[23] Little is known about the mechanisms of short-term potentiation,[23] so it will not be discussed here.
Each phase of LTP is governed by a set of mediators, small molecules that dictate the events of that phase.[4] These molecules include protein receptors that respond to events outside of the cell, enzymes that carry out chemical reactions within the cell, and signaling molecules that allow the progression from one phase to the next. In addition to these mediators, there are also modulator molecules, described later, that interact with mediators to finely alter the LTP ultimately generated.
The early (E-LTP) and late (L-LTP) phases of LTP are each characterized by a series of three events: induction, maintenance, and expression. Induction is the process by which a short-lived signal triggers that phase of LTP to begin. Maintenance corresponds to the persistent biochemical changes that occur in response to the induction of that phase. Expression entails the long-lasting cellular changes that result from activation of the maintenance signal.[23] Thus the mechanisms of LTP can be discussed in terms of the mediators that underlie the induction, maintenance, and expression of E-LTP and L-LTP.
Early phase
The early phase of LTP, one model of which is shown here, is independent of protein synthesis.[24]
Ca2+/calmodulin-dependent protein kinase II (CaMKII) appears to be an important mediator of the early, protein synthesis-independent phase of LTP.
Induction
- Main article: LTP induction
Induction of E-LTP occurs when the concentration of calcium inside the postsynaptic cell exceeds a critical threshold.[24] In many types of LTP, the flow of calcium into the cell requires the NMDA receptor, which is why these types of LTP are considered to be NMDA receptor-dependent.[24] NMDA receptor-dependent LTP can be induced experimentally by applying a few trains of high-frequency stimuli to the connection between two neurons.[25] An understanding of normal synaptic transmission illustrates how this tetanic stimulation can induce E-LTP.
Chemical synapses are functional connections between neurons throughout the nervous system. In a typical synapse, information is passed from the first (presynaptic) neuron to the second (postsynaptic) neuron via a process of synaptic transmission. Through experimental manipulation, a non-tetanic stimulus can be applied to the presynaptic cell, causing it to release a neurotransmitter—typically glutamate—onto the postsynaptic cell membrane. There, glutamate binds to AMPA receptors (AMPARs) embedded in the postsynaptic membrane. The AMPA receptor is one of the main excitatory receptors in the brain, and is responsible for most of its rapid, moment-to-moment excitatory activity.[26] Glutamate binding to the AMPA receptor triggers the influx of positively charged sodium ions into the postsynaptic cell, causing a short-lived depolarization called the excitatory postsynaptic potential (EPSP).
The magnitude of this depolarization determines whether E-LTP will be induced in the postsynaptic cell. While a single stimulus does not generate an EPSP capable of inducing E-LTP, repeated stimuli given at high frequency cause the postsynaptic cell to be progressively depolarized as a result of EPSP summation: with each EPSP reaching the postsynaptic cell before the previous EPSP can decay, successive EPSPs add to the depolarization caused by the previous EPSPs. In synapses that exhibit NMDA receptor-dependent LTP, sufficient depolarization unblocks NMDA receptors (NMDARs), receptors that allow calcium to flow into the cell when bound by glutamate. While NMDARs are present at most postsynaptic membranes, at resting membrane potentials they are blocked by a magnesium ion that prevents the entry of calcium into the postsynaptic cell. Sufficient depolarization through the summation of EPSPs relieves the magnesium blockade of the NMDAR, allowing calcium influx (despite the reduced driving force for calcium entry). The rapid rise in intracellular calcium concentration triggers the short-lasting activation of several enzymes that mediate E-LTP induction. Of particular importance are some protein kinase enzymes, including calcium/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC).[23] To a lesser extent, protein kinase A (PKA) and mitogen-activated protein kinase (MAPK) activation also contribute to the induction of E-LTP.[23]
Maintenance
While induction entails the transient activation of CaMKII and PKC, maintenance of E-LTP is characterized by their persistent activation. During this stage, PKMz(Protein kinase Mζ) which does not have dependence on calcium, become autonomously active. Consequently they are able to carry out the phosphorylation events that underlie E-LTP expression.[23]
Expression
Phosphorylation is a chemical reaction in which a small phosphate group is added to another molecule to change that molecule's activity. Autonomously active CaMKII and PKC use phosphorylation to carry out the two major mechanisms underlying the expression of E-LTP. First, and most importantly, they phosphorylate existing AMPA receptors to increase their activity.[4] Second, they mediate or modulate the insertion of additional AMPA receptors into the postsynaptic membrane.[4] Importantly, the delivery of AMPA receptors to the synapse during E-LTP is independent of protein synthesis. This is achieved by having a nonsynaptic pool of AMPA receptors adjacent to the postsynaptic membrane. When the appropriate LTP-inducing stimulus arrives, nonsynaptic AMPA receptors are rapidly trafficked into the postsynaptic membrane under the influence of protein kinases.[27] As mentioned previously, AMPA receptors are the brain's most abundant glutamate receptors and mediate the majority of its excitatory activity. By increasing the efficiency and number of AMPA receptors at the synapse, future excitatory stimuli generate larger postsynaptic responses.
While the above model of E-LTP describes entirely postsynaptic mechanisms for induction, maintenance, and expression, an additional component of expression may occur presynaptically.[28] One hypothesis of this presynaptic facilitation is that persistent CaMKII activity during E-LTP may lead to the synthesis of a "retrograde messenger", discussed later. According to this hypothesis, the newly synthesized messenger travels across the synaptic cleft from the postsynaptic to the presynaptic cell, leading to a chain of events that facilitate the presynaptic response to subsequent stimuli. Such events may include an increase in neurotransmitter vesicle number, probability of vesicle release, or both. In addition to the retrograde messenger underlying presynaptic expression in early LTP, the retrograde messenger may also play a role in the expression of late LTP.
Late phase
The early and late phases of LTP are thought to communicate via the extracellular signal-regulated kinase (ERK).[24]
Late LTP is the natural extension of E-LTP. Unlike E-LTP, which is independent of protein synthesis, L-LTP requires gene transcription[29] and protein synthesis[30] in the postsynaptic cell. Two phases of L-LTP exist: the first depends upon protein synthesis, while the second depends upon both gene transcription and protein synthesis.[24] These phases are occasionally called LTP2 and LTP3, respectively, with E-LTP referred to as LTP1 under this nomenclature.
Induction
Late LTP is induced by changes in gene expression and protein synthesis brought about by the persistent activation of protein kinases activated during E-LTP, such as MAPK.[23][24][31] In fact, MAPK—specifically the extracellular signal-regulated kinase (ERK) subfamily of MAPKs—may be the molecular link between E-LTP and L-LTP, since many signaling cascades involved in E-LTP, including CaMKII and PKC, can converge on ERK.[31] Recent research has shown that the induction of L-LTP can depend on coincident molecular events, namely PKA activation and calcium influx, that converge on CRTC1 (TORC1), a potent transcriptional coactivator for CREB.[32] This requirement for a molecular coincidence accounts perfectly for the associative nature of LTP, and, presumably, for that of learning.
Maintenance
Upon activation, ERK may phosphorylate a number of cytoplasmic and nuclear molecules that ultimately result in the protein synthesis and morphological changes observed in L-LTP.[24] These cytoplasmic and nuclear molecules may include transcription factors such as cAMP response element binding protein (CREB).[23] ERK-mediated changes in transcription factor activity may trigger the synthesis of proteins that underlie the maintenance of L-LTP. One such molecule may be protein kinase Mζ (PKMζ), a persistently active kinase whose synthesis increases following LTP induction.[33][34] PKMζ is an atypical isoform of PKC that lacks a regulatory subunit and thus remains constitutively active.[33] Unlike other kinases that mediate LTP, PKMζ is active not just in the first 30 minutes following LTP induction; rather, PKMζ becomes a requirement for LTP maintenance only during the late phase of LTP.[33] PKMζ thus appears important for the persistence of memory and would be expected to be important in the maintenance of long-term memory. Indeed, administration of a PKMζ inhibitor into the hippocampi of rats results in retrograde amnesia with intact short-term memory; PKMζ does not play a role in the establishment of short-term memory.[34] PKMζ has recently been shown to underlie L-LTP maintenance[33][34] by directing the trafficking and reorganization of proteins in the synaptic scaffolding that underlie the expression of L-LTP.[33]
Expression
Aside from PKMζ, the identities of only a few proteins synthesized during L-LTP are known. Regardless of their identities, it is thought that they contribute to the increase in dendritic spine number, surface area, and postsynaptic sensitivity to neurotransmitter associated with L-LTP expression.[24] The latter may be brought about in part by the enhanced synthesis of AMPA receptors during L-LTP.[24] Late LTP is also associated with the presynaptic synthesis of synaptotagmin and an increase in synaptic vesicle number, suggesting that L-LTP induces protein synthesis not only in postsynaptic cells, but in presynaptic cells as well.[24] As mentioned previously, for postsynaptic LTP induction to result in presynaptic protein synthesis, there must be communication from the postsynaptic to the presynaptic cell. This may occur via the synthesis of a retrograde messenger, discussed later.
Even in studies restricted to postsynaptic events, investigators have not determined the location of the protein synthesis that underlies L-LTP. Specifically, it is unclear whether protein synthesis takes place in the postsynaptic cell body or in its dendrites.[31] Despite having observed ribosomes (the major components of the protein synthesis machinery) in dendrites as early as the 1960s, prevailing wisdom was that the cell body was the predominant site of protein synthesis in neurons.[31] This reasoning was not seriously challenged until the 1980s, when investigators reported observing protein synthesis in dendrites whose connection to their cell body had been severed.[31] More recently, investigators have demonstrated that this type of local protein synthesis is necessary for some types of LTP.[35][36]
One reason for the popularity of the local protein synthesis hypothesis is that it provides a possible mechanism for the specificity associated with LTP.[31] Specifically, if indeed local protein synthesis underlies L-LTP, only dendritic spines receiving LTP-inducing stimuli will undergo LTP; the potentiation will not be propagated to adjacent synapses. By contrast, global protein synthesis that occurs in the cell body requires that proteins be shipped out to every area of the cell, including synapses that have not received LTP-inducing stimuli. Whereas local protein synthesis provides a mechanism for specificity, global protein synthesis would seem to directly compromise it. However, as discussed later, the synaptic tagging hypothesis successfully reconciles global protein synthesis, synapse specificity, and associativity.
Retrograde signaling
- Main article: Retrograde signaling in LTP
Retrograde signaling is a hypothesis that attempts to explain that, while LTP is induced and expressed postsynaptically, some evidence suggests that it is expressed presynaptically as well.[4][28][37] The hypothesis gets its name because normal synaptic transmission is directional and proceeds from the presynaptic to the postsynaptic cell. For induction to occur postsynaptically and be partially expressed presynaptically, a message must travel from the postsynaptic cell to the presynaptic cell in a retrograde (reverse) direction. Once there, the message presumably initiates a cascade of events that leads to a presynaptic component of expression, such as the increased probability of neurotransmitter vesicle release.[38]
Retrograde signaling is currently a contentious subject as some investigators do not believe the presynaptic cell contributes at all to the expression of LTP.[4] Even among proponents of the hypothesis there is controversy over the identity of the messenger. Early thoughts focused on nitric oxide, while most recent evidence points to cell adhesion proteins.[4]
Synaptic tagging
Before the local protein synthesis hypothesis gained significant support, there was general agreement that the protein synthesis underlying L-LTP occurred in the cell body. Further, there was thought that the products of this synthesis were shipped cell-wide in a nonspecific manner. It thus became necessary to explain how protein synthesis could occur in the cell body without compromising LTP's input specificity. The synaptic tagging hypothesis attempts to solve the cell's difficult problem of synthesizing proteins in the cell body but ensuring they only reach synapses that have received LTP-inducing stimuli.
The synaptic tagging hypothesis proposes that a "synaptic tag" is synthesized at synapses that have received LTP-inducing stimuli, and that this synaptic tag may serve to capture plasticity-related proteins shipped cell-wide from the cell body.[39] Studies of LTP in the marine snail Aplysia californica have implicated synaptic tagging as a mechanism for the input-specificity of LTP.[40][41] There is some evidence that given two widely separated synapses, an LTP-inducing stimulus at one synapse drives several signaling cascades (described previously) that initiates gene expression in the cell nucleus. At the same synapse (but not the unstimulated synapse), local protein synthesis creates a short-lived (less than three hours) synaptic tag. The products of gene expression are shipped globally throughout the cell, but are only captured by synapses that express the synaptic tag. Thus only the synapse receiving LTP-inducing stimuli is potentiated, demonstrating LTP's input specificity.
The synaptic tag hypothesis may also account for LTP's associativity and cooperativity. Associativity (see Properties) is observed when one synapse is excited with LTP-inducing stimulation while a separate synapse is only weakly stimulated. Whereas one might expect only the strongly stimulated synapse to undergo LTP (since weak stimulation alone is insufficient to induce LTP at either synapse), both synapses will in fact undergo LTP. While weak stimuli are unable to induce protein synthesis in the cell body, they may prompt the synthesis of a synaptic tag. Simultaneous strong stimulation of a separate pathway, capable of inducing cell body protein synthesis, then may prompt the production of plasticity-related proteins, which are shipped cell-wide. With both synapses expressing the synaptic tag, both would capture the protein products resulting in the expression of LTP in both the strongly stimulated and weakly stimulated pathways.
Cooperativity is observed when two synapses are activated by weak stimuli incapable of inducing LTP when stimulated individually. But upon simultaneous weak stimulation, both synapses undergo LTP in a cooperative fashion. Synaptic tagging does not explain how multiple weak stimuli can result in a collective stimulus sufficient to induce LTP (this is explained by the postsynaptic summation of EPSPs described previously). Rather, synaptic tagging explains the ability of weakly stimulated synapses, none of which are capable of independently generating LTP, to receive the products of protein synthesis initiated collectively. As before, this may be accomplished through the synthesis of a local synaptic tag following weak synaptic stimulation.
Modulation
| Modulator | Target |
|---|---|
| β-Adrenergic receptor | cAMP, MAPK amplification |
| Nitric oxide synthase | Guanylyl cyclase, PKG, NMDAR |
| Dopamine receptor | cAMP, MAPK amplification |
| Metabotropic glutamate receptor | PKC, MAPK amplification |
As described previously, the molecules that underlie LTP can be classified as mediators or modulators. A mediator of LTP is a molecule, such as the NMDA receptor or calcium, whose presence and activity is necessary for generating LTP under nearly all conditions. By contrast, a modulator is a molecule that can alter LTP but is not essential for its generation or expression.[4]
In addition to the signaling pathways described above, hippocampal LTP may be altered by a variety of modulators. For example, the steroid hormone estradiol may enhance LTP by driving CREB phosphorylation and subsequent dendritic spine growth.[42] Additionally, β-adrenergic receptor agonists such as norepinephrine may alter the protein synthesis-dependent late phase of LTP.[43] Nitric oxide synthase activity may also result in the subsequent activation of guanylyl cyclase and PKG.[44] Similarly, activation of dopamine receptors may enhance LTP through the cAMP/PKA signaling pathway.[45][46]
Relationship to behavioral memory
While the long-term potentiation of synapses in cell culture seems to provide an elegant substrate for learning and memory, the contribution of LTP to behavioral learning — that is, learning at the level of the whole organism — cannot simply be extrapolated from in vitro studies. For this reason, considerable effort has been dedicated to establishing whether LTP is a requirement for learning and memory in living animals.
Spatial memory

The Morris water maze task has been used to demonstrate the necessity of NMDA receptors in establishing spatial memories.
In 1986, Richard Morris provided some of the first evidence that LTP was indeed required for the formation of memories in vivo.[47] He tested the spatial memory of rats by pharmacologically modifying their hippocampus, a brain structure whose role in spatial learning is well established. Rats were trained on the Morris water maze, a spatial memory task in which rats swim in a pool of murky water until they locate the platform hidden beneath its surface. During this exercise, normal rats are expected to associate the location of the hidden platform with salient cues placed at specific positions around the circumference of the maze. After training, one group of rats had their hippocampi bathed in the NMDA receptor blocker APV, while the other group served as the control. Both groups were then subjected to the water maze spatial memory task. Rats in the control group were able to locate the platform and escape from the pool, while the performance of APV-treated rats was significantly impaired. Moreover, when slices of the hippocampus were taken from both groups, LTP was easily induced in controls, but could not be induced in the brains of APV-treated rats. This provided early evidence that the NMDA receptor — and by extension, LTP — was required for at least some types of learning and memory.
Similarly, Susumu Tonegawa demonstrated in 1996 that the CA1 area of the hippocampus is crucial to the formation of spatial memories in living mice.[48] So-called place cells located in this region become active only when the rat is in a particular location — called a place field — in the environment. Since these place fields are distributed throughout the environment, one interpretation is that groups of place cells form maps in the hippocampus. The accuracy of these maps determines how well a rat learns about its environment and thus how well it can navigate it. Tonegawa found that by impairing the NMDA receptor, specifically by genetically removing the NR1 subunit in the CA1 region, the place fields generated were substantially less specific than those of controls. That is, rats produced faulty spatial maps when their NMDA receptors were impaired. As expected, these rats performed very poorly on spatial tasks compared to controls, further supporting the role of LTP in spatial learning.
Enhanced NMDA receptor activity in the hippocampus has also been shown to produce enhanced LTP and an overall improvement in spatial learning. In 2001, Joe Tsien produced a line of mice with enhanced NMDA receptor function by overexpressing the NR2B subunit in the hippocampus.[49][50] The resulting smart mice, nicknamed "Doogie mice" after the fictional prodigious doctor Doogie Howser, had larger LTP and excelled at spatial learning tasks, reinforcing LTP's importance in the formation of hippocampus-dependent memories.
Inhibitory avoidance
In 2006, Jonathan Whitlock and colleagues reported on a series of experiments that provided perhaps the strongest evidence of LTP's role in behavioral memory, arguing that to conclude that LTP underlies behavioral learning, the two processes must both mimic and occlude one another.[51] Employing an inhibitory avoidance learning paradigm, researchers trained rats in a two-chambered apparatus with light and dark chambers, the latter being fitted with a device that delivered a foot shock to the rat upon entry. An analysis of CA1 hippocampal synapses revealed that inhibitory avoidance training induced in vivo AMPA receptor phosphorylation of the same type as that seen in LTP in vitro; that is, inhibitory avoidance training mimicked LTP. In addition, synapses potentiated during training could not be further potentiated by experimental manipulations that would have otherwise induced LTP; that is, inhibitory avoidance training occluded LTP. In a response to the article, Timothy Bliss and colleagues remarked that these and related experiments "substantially advance the case for LTP as a neural mechanism for memory."[52]
Clinical significance
The role of LTP in disease is less clear than its role in basic mechanisms of synaptic plasticity. However, alterations in LTP may contribute to a number of neurological diseases, including depression, Parkinson's disease, epilepsy, and neuropathic pain.[53] Impaired LTP may also have a role in Alzheimer's disease and drug addiction.
Alzheimer's disease
Misprocessing of amyloid precursor protein (APP) in Alzheimer's disease disrupts LTP and is thought to lead to early cognitive decline in individuals with the disease.[54]
LTP has received much attention among those who study Alzheimer's disease (AD), a neurodegenerative disease that causes marked cognitive decline and dementia. Much of this deterioration occurs in association with degenerative changes in the hippocampus and other medial temporal lobe structures. Because of the hippocampus' well established role in LTP, some have suggested that the cognitive decline seen in individuals with AD may result from impaired LTP.
In a 2003 review of the literature, Rowan et al. proposed one model for how LTP might be affected in AD.[54] AD appears to result, at least in part, from misprocessing of amyloid precursor protein (APP). The result of this abnormal processing is the accumulation of fragments of this protein, called amyloid β (Aβ). Aβ exists in both soluble and fibrillar forms. Misprocessing of APP results in the accumulation of soluble Aβ that, according to Rowan's hypothesis, impairs hippocampal LTP and may lead to the cognitive decline seen early in AD.
AD may also impair LTP through mechanisms distinct from Aβ. For example, one study demonstrated that the enzyme PKMζ accumulates in neurofibrillary tangles, which are a pathologic marker of AD. PKMζ is an enzyme with critical importance in the maintenance of late LTP.[55]
Drug addiction
Research in the field of addiction medicine has also recently turned its focus to LTP, owing to the hypothesis that drug addiction represents a powerful form of learning and memory.[56] Addiction is a complex neurobehavioral phenomenon involving various parts of the brain, such as the ventral tegmental area (VTA) and nucleus accumbens (NAc). Studies have demonstrated that VTA and NAc synapses are capable of undergoing LTP[56] and that this LTP may be responsible for the behaviors that characterize addiction.[57]
See also
References
- ↑ Paradiso, Michael A.; Bear, Mark F.; Connors, Barry W. (2007). Neuroscience: Exploring the Brain, Hagerstwon, MD: Lippincott Williams & Wilkins. URL accessed 2008-12-25.
- ↑ 2.0 2.1 2.2 2.3 Cooke SF, Bliss TV (2006). Plasticity in the human central nervous system. Brain 129 (Pt 7): 1659–73.
- ↑ 3.0 3.1 Bliss TV, Collingridge GL (January 1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361 (6407): 31–9.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Malenka R, Bear M (2004). LTP and LTD: an embarrassment of riches. Neuron 44 (1): 5–21.
- ↑ Williams RW, Herrup K (1988). The control of neuron number. Annu. Rev. Neurosci. 11: 423–53.
- ↑ 6.0 6.1 Ramón y Cajal, Santiago (1894). The Croonian Lecture: La Fine Structure des Centres Nerveux. Proceedings of the Royal Society of London 55: 444–468.
- ↑ Hebb, D. O. (1949). Organization of Behavior: a Neuropsychological Theory, New York: John Wiley.
- ↑ 8.0 8.1 Terje Lømo (2003). The discovery of long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358 (1432): 617–20.
- ↑ Lømo, Terje (1966). Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation. Acta Physiologica Scandinavica 68 (Suppl 277): 128.
- ↑ 10.0 10.1 Bliss T, Lømo T (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232 (2): 331–56.
- ↑ 11.0 11.1 Bliss T, Gardner-Medwin A (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J. Physiol. (Lond.) 232 (2): 357–74.
- ↑ While the term "long term potentiation" appeared once in the original Bliss and Lømo paper, it was not formally proposed for the phenomenon until the Douglas and Goddard paper.
- ↑ Douglas R, Goddard G (1975). Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 86 (2): 205–15.
- ↑ Andersen P (2003). A prelude to long-term potentiation. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 358 (1432): 613–5.
- ↑ Clugnet, MC, LeDoux JE (1 August 1990). Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body.. J Neurosci 10 (8): 2818–24.
- ↑ Yasuda H, Barth A, Stellwagen D, Malenka R (2003). A developmental switch in the signaling cascades for LTP induction. Nat Neurosci 6 (1): 15–6.
- ↑ Harris E, Cotman C (1986). Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett 70 (1): 132–7.
- ↑ Wigström H, Gustafsson B (1986). Postsynaptic control of hippocampal long-term potentiation. J. Physiol. (Paris) 81 (4): 228–36.
- ↑ Urban NN, Barrionuevo G (July 1996). Induction of hebbian and non-hebbian mossy fiber long-term potentiation by distinct patterns of high-frequency stimulation. J. Neurosci. 16 (13): 4293–9.
- ↑ Kullmann DM, Lamsa K (March 2008). Roles of distinct glutamate receptors in induction of anti-Hebbian long-term potentiation. J. Physiol. (Lond.) 586 (6): 1481–6.
- ↑ McNaughton BL (April 2003). Long-term potentiation, cooperativity and Hebb's cell assemblies: a personal history. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 358 (1432): 629–34.
- ↑ Abraham WC (April 2003). How long will long-term potentiation last?. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 358 (1432): 735–44.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 Sweatt J (1999). Toward a molecular explanation for long-term potentiation. Learn Mem 6 (5): 399–416.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 24.8 24.9 Lynch M (2004). Long-term potentiation and memory. Physiol Rev 84 (1): 87–136.
- ↑ Huang Y, Kandel E (1994). Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem 1 (1): 74–82.
- ↑ Agranoff, Bernard W.; Siegel, George J. (1999). Basic neurochemistry: molecular, cellular, and medical aspects, 326, Philadelphia: Lippincott-Raven.
- ↑ Malinow R (2003). AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358 (1432): 707–14.
- ↑ 28.0 28.1 Emptage N, Reid C, Fine A, Bliss T (2003). Optical quantal analysis reveals a presynaptic component of LTP at hippocampal Schaffer-associational synapses. Neuron 38 (5): 797–804.
- ↑ Frey U, Frey S, Schollmeier F, Krug M (1 January 1996). Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol 490 (Pt 3) (Pt 3): 703–11.
- ↑ Frey U, Krug M, Reymann K, Matthies H (1988). Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res 452 (1-2): 57–65.
- ↑ 31.0 31.1 31.2 31.3 31.4 31.5 Kelleher R, Govindarajan A, Tonegawa S (2004). Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 44 (1): 59–73.
- ↑ Kovács KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR (2007). TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity.. PNAS 104 (11): 4700–5.
- ↑ 33.0 33.1 33.2 33.3 33.4 Serrano P, Yao Y, Sacktor T (2005). Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. J Neurosci 25 (8): 1979–84.
- ↑ 34.0 34.1 34.2 Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton A, Sacktor T (2006). Storage of spatial information by the maintenance mechanism of LTP. Science 313 (5790): 1141–4.
- ↑ Kang H, Schuman E (1996). A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science 273 (5280): 1402–6.
- ↑ Steward O, Worley P (2001). A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci USA 98 (13): 7062–8.
- ↑ Pavlidis P, Montgomery J, Madison D (2000). Presynaptic protein kinase activity supports long-term potentiation at synapses between individual hippocampal neurons. J Neurosci 20 (12): 4497–505.
- ↑ Zakharenko S, Patterson S, Dragatsis I, Zeitlin S, Siegelbaum S, Kandel E, Morozov A (2003). Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron 39 (6): 975–90.
- ↑ Frey U, Morris R (1997). Synaptic tagging and long-term potentiation. Nature 385 (6616): 533–6.
- ↑ Martin K, Casadio A, Zhu H, Yaping E, Rose J, Chen M, Bailey C, Kandel E (1997). Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91 (7): 927–38.
- ↑ Casadio A, Martin K, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey C, Kandel E (1999). A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99 (2): 221–37.
- ↑ Segal M, Murphy D (1999). CREB activation mediates plasticity in cultured hippocampal neurons. Neural Plast 6 (3): 1–7.
- ↑ Straube T, Frey J (2003). Involvement of beta-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength. Neuroscience 119 (2): 473–9.
- ↑ Lu Y, Kandel E, Hawkins R (1999). Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci 19 (23): 10250–61.
- ↑ Frey U, Matthies H, Reymann K, Matthies H (1991). The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci Lett 129 (1): 111–4.
- ↑ Otmakhova N, Lisman J (1996). D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci 16 (23): 7478–86.
- ↑ Morris R, Anderson E, Lynch G, Baudry M (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319 (6056): 774–6.
- ↑ McHugh T, Blum K, Tsien J, Tonegawa S, Wilson M (1996). Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87 (7): 1339–49.
- ↑ Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ (1999). Genetic enhancement of learning and memory in mice. Nature 401: 63–69.
- ↑ Tang Y, Wang H, Feng R, Kyin M, Tsien J (2001). Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 41 (6): 779–90.
- ↑ Whitlock J, Heynen A, Shuler M, Bear M (2006). Learning induces long-term potentiation in the hippocampus. Science 313 (5790): 1093–7.
- ↑ Bliss T, Collingridge G, Laroche S (2006). Neuroscience. ZAP and ZIP, a story to forget. Science 313 (5790): 1058–9.
- ↑ Cooke SF, Bliss TV (July 2006). Plasticity in the human central nervous system. Brain: A Journal of Neurology 129 (Pt 7): 1659–73.
- ↑ 54.0 54.1 Rowan MJ, Klyubin I, Cullen WK, Anwyl R (April 2003). Synaptic plasticity in animal models of early Alzheimer's disease. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 358 (1432): 821–8.
- ↑ Crary JF, Shao CY, Mirra SS, Hernandez AI, Sacktor TC (April 2006). Atypical protein kinase C in neurodegenerative disease I: PKMzeta aggregates with limbic neurofibrillary tangles and AMPA receptors in Alzheimer disease. Journal of neuropathology and experimental neurology 65 (4): 319–26.
- ↑ 56.0 56.1 Kauer JA, Malenka RC (November 2007). Synaptic plasticity and addiction. Nature reviews. Neuroscience 8 (11): 844–58.
- ↑ Wolf ME (August 2003). LTP may trigger addiction. Molecular interventions 3 (5): 248–52.
Further reading
- Bliss, T; Collingridge, G; Morris, R (2004). Long-term potentiation: enhancing neuroscience for 30 years, Oxford: Oxford University Press.
External links
- Researchers provide first evidence for learning mechanism, a PhysOrg.com report on 2006 study by Bear and colleagues.
- Short video documentary about the Doogie mice. (RealPlayer format)
- "Smart Mouse", a Quantum ABC TV episode about the Doogie mice.
- MeSH Long-Term+Potentiation
Nervous system physiology: neurophysiology / clinical neurophysiology | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primarily CNS |
Arousal (Wakefulness) · Intracranial pressure · Lateralization of brain function · Sleep · Memory | ||||||||||
| Primarily PNS | |||||||||||
| Both |
| ||||||||||
| {| class="navbox collapsible nowraplinks" style="margin:auto; " | |||||||||||
| |||||||||||
|
|}
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |