| Line 42: | Line 42: | ||
===Effects=== |
===Effects=== |
||
| + | |||
| + | {{Physiological effects of cortisol}} |
||
| + | |||
:''See also [[Glucocorticoid#Medical uses and effects of high-dose glucocorticoids|Medical uses and effects of high dose glucocorticoids]]'' |
:''See also [[Glucocorticoid#Medical uses and effects of high-dose glucocorticoids|Medical uses and effects of high dose glucocorticoids]]'' |
||
| + | In normal release, cortisol (like other [[glucocorticoid]] agents) has widespread actions which help restore [[homeostasis]] after [[Stress (medicine)|stress]]. (These normal [[endogenous]] functions are the basis for the physiological consequences of chronic stress - prolonged cortisol secretion.). It has been proposed that its primary function is to inversely mobilize the immune system to fight potassium losing diarrhea diseases <ref>Weber CE (1998) “Cortisol’s purpose.” Medical Hypotheses 51; 289-292.</ref>. Its odd attributes all support this. |
||
| + | |||
| + | ==l== |
||
In normal release, cortisol (like other [[glucocorticoid]] agents) has widespread actions which help restore [[homeostasis]] after [[Stress (medicine)|stress]]. (These normal [[endogenous]] functions are the basis for the physiological consequences of chronic stress - prolonged cortisol secretion.). It has been proposed that its primary function is to inversely mobilize the immune system to fight potassium losing diarrhea diseases <ref>Weber CE (1998) “Cortisol’s purpose.” Medical Hypotheses 51; 289-292.</ref>. Its odd attributes all support this. |
In normal release, cortisol (like other [[glucocorticoid]] agents) has widespread actions which help restore [[homeostasis]] after [[Stress (medicine)|stress]]. (These normal [[endogenous]] functions are the basis for the physiological consequences of chronic stress - prolonged cortisol secretion.). It has been proposed that its primary function is to inversely mobilize the immune system to fight potassium losing diarrhea diseases <ref>Weber CE (1998) “Cortisol’s purpose.” Medical Hypotheses 51; 289-292.</ref>. Its odd attributes all support this. |
||
Revision as of 23:42, 22 January 2010
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
|
Hydrocortisone chemical structure | |
| 11,17,21-trihydroxy-,(11beta)- pregn-4-ene-3,20-dione IUPAC name | |
| CAS number 50-23-7 |
ATC code H02AB09 (and others) |
| PubChem 5754 |
DrugBank [1] |
| Chemical formula | |
| Molecular weight | 362.465 |
| Bioavailability | |
| Metabolism | |
| Elimination half-life | |
| Excretion | {{{excretion}}} |
| Pregnancy category | C |
| Legal status | |
| Routes of administration | Oral tablets, intravenously, topical |
Cortisol is a corticosteroid hormone produced by the zona fasciculata of the adrenal cortex (in the adrenal gland). It is a vital hormone that is often referred to as the "stress hormone" as it is involved in the response to stress.
- Main article: Cortisol and stress
As it has a number of physiological effects such as increasing blood pressure, and blood sugar levels and has an immunosuppressive action it can be seen as a mediator between physical] and psychological stress and a number of psychosomatic disorders.
In pharmacology, the synthetic form of cortisol is referred to as hydrocortisone, and is used as an antagonist in the treatment of allergies and inflammation as well as substitute supplementation in cortisol production deficiencies. When first introduced as a treatment for rheumatoid arthritis, it was referred to as Compound E.
Physiology
The amount of cortisol present in the serum undergoes diurnal variation, with the highest levels present in the early morning, and the lowest levels present around midnight, 3-5 hours after the onset of sleep. Information about the light/dark cycle is transmitted from the retina to the paired suprachiasmatic nuclei in the hypothalamus. The pattern is not present at birth (estimates of when it starts vary from two weeks to 9 months).[1]
Changed patterns of serum cortisol levels have been observed in connection with abnormal ACTH levels, clinical depression, psychological stress, and such physiological stressors as hypoglycemia, illness, fever, trauma, surgery, fear, pain, physical exertion or extremes of temperature.
There is also significant individual variation, although a given person tends to have consistent rhythms.
Effects
Template:Physiological effects of cortisol
In normal release, cortisol (like other glucocorticoid agents) has widespread actions which help restore homeostasis after stress. (These normal endogenous functions are the basis for the physiological consequences of chronic stress - prolonged cortisol secretion.). It has been proposed that its primary function is to inversely mobilize the immune system to fight potassium losing diarrhea diseases [2]. Its odd attributes all support this.
l
In normal release, cortisol (like other glucocorticoid agents) has widespread actions which help restore homeostasis after stress. (These normal endogenous functions are the basis for the physiological consequences of chronic stress - prolonged cortisol secretion.). It has been proposed that its primary function is to inversely mobilize the immune system to fight potassium losing diarrhea diseases [3]. Its odd attributes all support this.
- Insulin
- It acts as a physiological antagonist to insulin by decreasing glycogenesis (formation of glycogen) and promotes breakdown of lipids (lipolysis), and proteins, and mobilization of extrahepatic amino acids and ketone bodies. This leads to increased circulating glucose concentrations (in the blood) by increasing gluconeogenesis. There is an increased glycogen breakdown in the liver . [4] Prolonged cortisol secretion causes hyperglycemia. Cortisol has no effect on insulin [5]. The reason why in vivo experiments seem to deny this is that cortisone greatly inhibits insulin . So the cortisone-cortisol equilibrium may explain why in vivo experiments contradict the cortisol effect [6]. Cortisol does cause serum glucose to rise, but this is probably an indirect effect caused by stimulation of amino acid degradation, especially that derived from collagen in the skin. Loss of collagen from skin by cortisol is ten times greater than from all other tissue in the rat. [7].
- Amino acids
- Cortisol raises the free amino acids in the serum. It does this by inhibiting collagen formation, decreasing amino acid uptake by muscle, and inhibiting protein synthesis.[8] Cortisol (as opticortinol) probably inversely inhibits IgA precursor cells in the intestines of calves [9]. Cortisol also inhibits IgA in serum, as it does IgM, but not IgE.[10]
- Gastric secretion
- Cortisol stimulates gastric acid secretion [11]. Gastric acid secretion would increase loss of potassium into the stomach during diarrhea as well as acid loss. Cortisol's only direct effect on the hydrogen ion excretion of the kidneys is to stimulate excretion of ammonium ion by inactivation of renal glutaminase enzyme [12]. Net chloride secretion in the intestines is inversely decreased by cortisol in vitro (methylprednisolone) [13].
- Sodium
- Cortisol inhibits loss of sodium from small intestines of mammals. [14]. However sodium depletion does not affect cortisol [15], so cortisol is not used to regulate serum sodium. Cortisol’s purpose may originally had been centered around moving sodium because cortisol is used to stimulate sodium inward for fresh water fish and outward for salt-water fish [16].
- Potassium
- Sodium loads augments the intense potassium excretion by cortisol, and corticosterone is comparable to cortisol in this case [17]. In order for potassium to move out of the cell, cortisol moves in an equal number of sodium ions [18]. It can be seen that this should make pH regulation much easier, unlike the normal potassium deficiency situation in which about 2 sodium ions move in for each 3 potassium ions that move out, which is closer to the deoxycorticosterone effect. Nevertheless, cortisol consistently causes alkalosis of the serum, while in a deficiency pH does not change. Perhaps this may be for the purpose of bringing serum pH to a value most optimum for some of the immune enzymes during infection in those times when cortisol declines. Potassium is also blocked from loss in the kidneys directly somewhat by decline of cortisol (9 alpha fluorohydrocortisone) [19].
- Water
- Cortisol also acts as a water diuretic hormone. Half the intestinal diuresis is so controlled [20]. Kidney diuresis is also controlled by cortisol in dogs. The decline in water excretion upon decline of cortisol (dexamethasone) in dogs is probably due to inverse stimulation of antidiuretic hormone (ADH or arginine vasopressin), the inverse stimulation of which is not overridden by water loading.[21]. Humans also use this mechanism [22] and other different animal mechanisms operate in the same direction.
- Copper
- It is probable that increasing copper availability for immune purposes is the reason why many copper enzymes are stimulated to an extent which is often 50% of their total potential by cortisol [23]. This includes lysyl oxidase, an enzyme which is used to cross link collagen and elastin [24]. Particularly valuable for immunity is the stimulation of superoxide dismutase by cortisol [25] since this copper enzyme is almost certainly used by the body to permit superoxide to poison bacteria. Cortisol causes an inverse four or five fold decrease of metallothionein, a copper storage protein, in mice [26] (however rodents do not synthesize cortisol themselves),. This may be to furnish more copper for ceruloplasmin synthesis or release of free copper. Cortisol has an opposite effect on alpha aminoisobuteric acid than on the other amino acids [27]. If alpha aminoisobuteric acid is used to transport copper through the cell wall, this anomaly would possibly be explained.
- Immune system
- Cortisol can weaken the activity of the immune system . Cortisol prevents proliferation of T-cells by rendering the interleukin-2 producer T-cells unresponsive to interleukin-1 (IL-1), and unable to produce the T-cell growth factor.[28] Cortisol has a negative feedback effect on interleukin-1 [29] which must be especially useful in combating diseases, such as the endotoxin bacteria, that gain an advantage by forcing the hypothalamus to secrete a hormone called CRH. The suppressor cells are not affected by GRMF, [30] so that the effective set point for the immune cells may be even higher than the set point for physiological processes. It reflects leukocyte redistribution to lymph nodes, bone marrow, and skin. Acute administration of corticosterone (the endogenous Type I and Type II receptor agonist), or RU28362 (a specific Type II receptor agonist), to adrenalectomized animals induced changes in leukocyte distribution. Natural killer cells are not affected by cortisol [31].
- Bone metabolism
- It lowers bone formation thus favoring development of osteoporosis in the long term. Cortisol moves potassium out of cells in exchange for an equal number of sodium ions as mentioned above.[32] This can cause a major problem with the hyperkalemia of metabolic shock from surgery.
- Memory
- It cooperates with epinephrine (adrenaline) to create memories of short-term emotional events; this is the proposed mechanism for storage of flash bulb memories, and may originate as a means to remember what to avoid in the future. However, long-term exposure to cortisol results in damage to cells in the hippocampus. This damage results in impaired learning. The desirability of inhibiting activity during infection is no doubt the reason why cortisol is responsible for creating euphoria, [33]. The desirability of not disturbing tissues weakened by infection or of not cutting off their blood supply could explain the inhibition of pain widely observed for cortisol.
- Additional effects
- It increases blood pressure by increasing the sensitivity of the vasculature to epinephrine and norepinephrine. In the absence of cortisol, widespread vasodilation occurs.
- It inhibits the secretion of corticotropin-releasing hormone (CRH), resulting in feedback inhibition of ACTH secretion. Some researchers believe that this normal feedback system may break down when animals are exposed to chronic stress.
- It increases the effectiveness of catecholamines.
- It allows for the kidneys to produce hypotonic urine.
- It has anti-inflammatory effects by reducing histamine secretion and stabilizing lysosomal membranes. The stabilization of lysosomal membranes prevents their rupture, thereby preventing damage to healthy tissues.
- It stimulates hepatic detoxification by inducing tryptophan oxygenase (to reduce serotonin levels in the brain), glutamine synthase (reduce glutamate and ammonia levels in the brain), cytochrome P-450 hemoprotein (mobilizes arachidonic acid), and metallothionein (reduces heavy metals in the body).
- In addition to the effects caused by cortisol binding to the glucocorticoid receptor, because of its molecular similarity to aldosterone, it also binds to the mineralocorticoid receptor. (It binds with less affinity to it than aldosterone does, but the concentration of blood cortisol is higher than that of blood aldosterone.)
Binding
Most serum cortisol, all but about 4%, is bound to proteins including corticosteroid binding globulin (CBG), and serum albumin. Only free cortisol is available to most receptors.
Regulation
The primary control of cortisol is the pituitary gland peptide, adrenocorticotropic hormone (ACTH). ACTH probably controls cortisol by controlling movement of calcium into the cortisol secreting target cells.[34]. ACTH is in turn controlled by the hypothalamic peptide, corticotropin releasing factor (CRF), which is under nervous control. CRF acts synergisticly with arginine vasopressin, angiotensin II, and epinephrine [35]. When activated macrophages start to secrete interleukin-1 (IL-1), which synergistically with CRF increases ACTH, [36] T-cells also secrete glucosteroid response modifying factor (GRMF or GAF) as well as IL-1, both of which increase the amount of cortisol required to inhibit almost all the immune cells [37]. Thus immune cells take over their own regulation, but at a higher cortisol set point. Even so, the rise of cortisol in diarrheic calves is minimal over healthy calves and drops below with time. [38] The cells do not lose all of the fight or flight override because of interleukin-1's synergism with CRF. Cortisol even has a negative feedback effect on interleukin-1 [39] which must be especially useful against those diseases which gain an advantage by forcing the hypothalamus to secrete too much CRF, such as the endotoxin bacteria..The suppressor immune cells are not affected by GRMF, [40] so that the effective set point for the immune cells may be even higher than the set point for physiological processes. GRMF (called GAF in this reference) primarily affects the liver rather than the kidneys for some physiological processes [41].
A high potassium media, which stimulates aldosterone secretion in vitro, also stimulates cortisol secretion from the fasciculata zone of dog adrenals [42] unlike corticosterone, upon which potassium has no affect [43]. Potassium loading increases ACTH and cortisol in people also [44]. This is no doubt the reason why a potassium deficiency causes cortisol to decline (as just mentioned) and why a potassium deficiency causes a decrease in conversion of 11deoxycortisol to cortisol [45]. This probably contributes to the pain in rheumatoid arthritis since cell potassium is always low in that disease [46]
Diseases and disorders
- Hypercortisolism: Excessive levels of cortisol in the blood result in Cushing's syndrome.
- Hypocortisolism, or adrenal insufficiency: If on the other hand the adrenal glands do not produce sufficient amounts of cortisol, Addison's disease is the consequence.
The relationship between cortisol and ACTH is as follows:
| Plasma Cortisol | Plasma ACTH | |
|---|---|---|
| Primary Hypercortisolism (Cushing's syndrome) | ↑ | ↓ |
| Secondary Hypercortisolism (pituitary, Cushing's disease) | ↑ | ↑ |
| Primary Hypocortisolism (Addison's disease) | ↓ | ↑ |
| Secondary Hypocortisolism (pituitary) | ↓ | ↓ |
Pharmacology
Hydrocortisone is the chemical form of cortisol used for oral administration or intravenous injection. It is used as an immunosuppressive drug, given by injection in the treatment of severe allergic reactions such as anaphylaxis and angioedema, in place of prednisolone in patients who need steroid treatment but cannot take oral medication, and peri-operatively in patients on long-term steroid treatment to prevent an Addisonian crisis.
It may be used topically for allergic rashes, eczema, psoriasis and certain other inflammatory skin conditions. It may also be injected into inflamed joints resulting from diseases such as gout.
Compared to prednisolone, hydrocortisone is about 1/4 the strength for the anti-inflammatory effect, while Dexamethasone is about 40 times as strong as hydrocortisone. For side effects, see corticosteroid and prednisolone.
Hydrocortisone creams and ointments are available without prescription in strengths ranging from 0.5% to 2.5%, depending on local regulations, with stronger forms available with prescriptions only.
Biochemistry
Biosynthesis
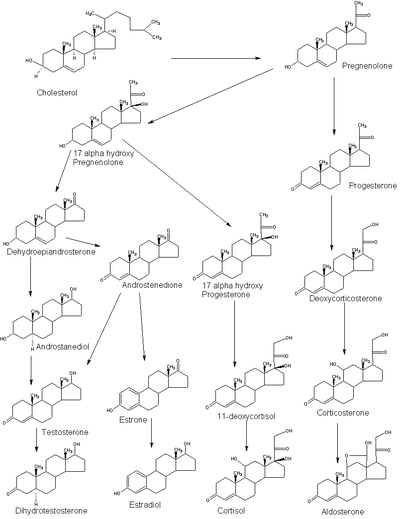
Steroidogenesis, showing cortisol at right.
Cortisol is synthesized from cholesterol. The synthesis takes place in the zona fasciculata of the cortex of the adrenal glands. (The name cortisol comes from cortex.) While the adrenal cortex also produces aldosterone (in the zona glomerulosa) and some sex hormones (in the zona reticularis), cortisol is its main secretion. The medulla of the adrenal gland lies under the cortex and mainly secretes the catecholamines, adrenaline (epinephrine) and noradrenaline (norepinephrine) under sympathetic stimulation (more epinephrine is produced than norepinephrine, in a ratio 4:1).
The synthesis of cortisol in the adrenal gland is stimulated by the anterior lobe of the pituitary gland with adrenocorticotropic hormone (ACTH); production of ACTH is in turn stimulated by corticotropin-releasing hormone (CRH), released by the hypothalamus. ACTH increases the concentration of cholesterol in the inner mitochondrial membrane (via regulation of STAR (steroidogenic acute regulatory) protein). The cholesterol is converted to pregnenolone, catalysed by Cytochrome P450SCC (side chain cleavage).
Metabolism
Cortisol is metabolized by the 11-beta hydroxysteroid dehydrogenase system (11-beta HSD), which consists of two enzymes: 11-beta HSD1 and 11-beta HSD2.
- 11-beta HSD1 utilizes the cofactor NADPH to convert biologically inert cortisone to biologically active cortisol.
- 11-beta HSD2 utilizes the cofactor NAD+ to convert cortisol to cortisone.
Overall the net effect is that 11-beta HSD1 serves to increase the local concentrations of biologically active cortisol in a given tissue, while 11-beta HSD2 serves to decrease the local concentrations of biologically active cortisol. The CA3 area of hippocampus (memory) is affected by cortisol. [How to reference and link to summary or text]
An alteration in 11-beta HSD1 has been suggested to play a role in the pathogenesis of obesity, hypertension, and insulin resistance, sometimes referred to the metabolic syndrome. [How to reference and link to summary or text]
An alteration in 11-beta HSD2 has been implicated in essential hypertension and is known to lead to the syndrome of apparent mineralocorticoid excess (SAME).[How to reference and link to summary or text]
See also
- Cushing's syndrome
- HPA axis
- ACTH stimulation test
- Hypopituitarism
- Post-traumatic stress disorder
- Central serous retinopathy
- CortiSlim
- Relacore, a pill which claims to reduce Cortisol.
Additional images
References
- ↑ de Weerth C, Zijl R, Buitelaar J (2003). Development of cortisol circadian rhythm in infancy. Early Hum Dev 73 (1-2): 39-52.
- ↑ Weber CE (1998) “Cortisol’s purpose.” Medical Hypotheses 51; 289-292.
- ↑ Weber CE (1998) “Cortisol’s purpose.” Medical Hypotheses 51; 289-292.
- ↑ Freeman, Scott (2002). Biological Science. Prentice Hall; 2nd Pkg edition (December 30, 2004). ISBN 0-13-218746-9.
- ↑ Barseghian, G.; Rachmiel, L.; Epps, P. (1982) “Direct Effect of Cortisol and Cortisone on Insulin and Glucagon Secretion”. Endocrinology 111: 1648,.
- ↑ Curry, D.L.; Bennett, L.L. (1973) “Dynamics of Insulin Release by Perfused Rate Pancreas: Effects of Hypophysectomy, Growth Hormone, Adrenocorticotropic Hormone and Hydrocortisone”. Endocrinology 93: 602,.
- ↑ Houck, J.C.; Sharma, V.K.; Patel, Y.M.; Gladner, J.A. (1968) “Induction of Collagenolytic and Proteolytic Activities by AntiInflammatory Drugs in the Skin and Fibroblasts”. Biochemical Pharmacology 17: 2081,
- ↑ Manchester, K.L., “Sites of Hormonal Regulation of Protein Metabolism. p. 229”, Mammalian Protein [Munro, H.N., Ed.]. Academic Press, New York. On p273.
- ↑ Husband, A.J.; Brandon, M.R.; Dascelles, A.K. (1973) “The Effect of Corticosteroid on Absorption and Endogenous Production of Immunoglobulins in Calves”. Aust. Journal of Exp. Biol. Med. Sci. 55: 707,.
- ↑ Posey, W.C.; Nelson, H.S.; Branch, B. and Pearlman, D.S. (1978) “The Effects of Acute Corticosteroid Therapy for Asthma on Serum Immunoglobulin Levels”. J. Allergy Clin. Immunol. 62: 340,.
- ↑ Soffer, L.J.; Dorfman, R.I.; Gabrilove, J.L,. “The Human Adrenal Gland”. Febiger, Phil.
- ↑ Kokshchuk, G.I.; Pakhmurnyi, B.A. (1979) “Role of Glucocorticoids in Regulation of the Acid-Excreting Function of the Kidneys”. Fiziol. Z H SSR I.M.I.M. Sechenova 65: 751,.
- ↑ Tai, Y.; Decker, R.A.; Marnane, W.G.; Charney, A.N.; Donowitz, M. (1981) "Effects of Methylprednisolone on Electrolyte Transport by Rat Ileum in Vitro." American Journal of Physiology 240-G346: 70,.
- ↑ Sandle, G.I.; Keir, M.G.; Record, CO. (1981) “The Effect of Hydrocortisone on the Transport of Water, Sodium, and Glucose in the Jejunum”. Scandinavian Journal of Gastroenterol. 16: 667,.
- ↑ Mason, P.A.; Fraser, R.; Morton, J.J. (1977) “The Effect of Sodium Deprivation and of Angiotensin II Infusion on the Peripheral Plasma Concentration of 18 Hydroxycorticosterone, Aldosterone, and Other Corticosteoids in Man”. Steroid Biochemistry 8: 799,
- ↑ Gorbman, A.; Dickhoff, W.W.; Vigna, S.R.; Clark, N.B.; Muller, A.F,. “Comparative Endocrinology”. John Wiley and Sons, New York.
- ↑ Muller AF Oconnor CM, ed. (1958) “An International Symposium on Aldosterone”, page 58. Little Brown & Co.
- ↑ Knight, R.P., Jr.; Kornfield, D.S.; Glaser, G.H. & Bondy, P.K. (1955) “Effects of Intravenous Hydrocortisone on Electrolytes in Serum and Urine in Man”. Journal of Clinical Endocrinology 15: 176-181,.
- ↑ Barger, A.C.; Berlin, R.D.; Tulenko, J.F. (1958) “Infusion of Aldosterone, 9 Alpha Fluorohydrocortisone, and Antidiuretic Hormone into the Renal Artery of Normal and Adrenalectomized Unanesthetized Dogs: Effect on Electrolyte and Water Excretion”. Endocrine. 62: 804,.
- ↑ Sandle, G.I.; Keir, M.G.; Record, CO. (1981) “The Effect of Hydrocortisone on the Transport of Water, Sodium, and Glucose in the Jejunum”. Scandinavian Journal of Gastroenterol. 16: 667,.
- ↑ Boykin, J.; de Torrent, A.; Erickson, A.; Robertson, G.; Schrier, R.W. (1978) “Role of Plasma Vasopressin in Impaired Water Excretion of Glucocorticoid Deficiency”. Journal of Clinical Investigation 62: 738,.
- ↑ Dingman, J.F.; Gonzalez-Auvert Ahmed, A.B.J.; Akinura, A. (1965) “Antidiuretic Hormone in Adrenal Insufficiency”. Journal of Clinical Investigation 44: 1041,.
- ↑ Weber, C.E (1984). “Copper Response to Rheumatoid Arthritis”. Medical Hypotheses 15: 333-348, on p337,.
- ↑ Weber, C.E. (1984) “Copper Response to Rheumatoid Arthritis”. Medical Hypotheses 15: 333,.on p334.
- ↑ Flohe, L.; Beckman, R.; Giertz, H.; Loschen, G. “Oxygen Centered Free Radicals as Mediators of Inflammation. p. 405”, Oxidative Stress (Sies H, ed) Academic Press, New York.
- ↑ Piletz, J.E.; Herschman, H.R. (1983) “Hepatic Metallothionein Synthesis in Neonatal Mottled-Brindled Mice”. Biochem. Genet. 21: 465.
- ↑ Chambers, J.W.; Georg, R.H. and Bass, A.D. (1965) “Effect of Hydrocortisone and Insulin on Uptake of Alpha Aminoisobutyric Acid by Isolated Perfused Rat Liver”. Mol. Pharmacol. 1: 66,.
- ↑ Palacios R., Sugawara I. (1982). Hydrocortisone abrogates proliferation of T cells in autologous mixed lymphocyte reaction by rendering the interleukin-2 Producer T cells unresponsive to interleukin-1 and unable to synthesize the T-cell growth factor. Scand J Immunol 15 (1): 25-31.
- ↑ Besedovsky, H.O.; Del Rey, A.; Sorkin, E. (1984) "Integration of Activated Immune Cell Products in Immune Endocrine Feedback Circuits." p. 200 in Leukocytes and Host Defense Vol. 5 [Oppenheim, J.J.; Jacobs, D.M., eds]. Alan R. Liss, New York,.
- ↑ Fairchild, S.L.; Shannon, K.; Kwan, E.; Mishell, R.I. (1984) "T-Cells Derived Glucocorticosteroid Lymphocytes and a T-Cell Hybridoma." Journal of Immunology 132: 821,
- ↑ Onsrud M Thorsby E (1981) “Influence of in vivo hydrocortisone on some blood lymphocyte subpopulations 1. effect on natural killer cell activity”. Scand. J. Immunol. 13; 573-579.
- ↑ Knight, R.P., Jr. Kornfield, D.S. Glaser, G.H. Bondy, P.K. (1955). Effects of intravenous hydrocortisone on electrolytes of serum and urine in man. J Clin Endocrinol Metab 15 (2): 176-81.
- ↑ Newsholme, E.A., Leech, A.R. “Biochemistry for the Medical Sciences. John Wiley & Sons, New York, on p736.
- ↑ Davies E. Keyon, C.J.; Fraser, R. (1985) "The role of calcium ions in the mechanism of ACTH stimulation of cortisol synthesis." Steroids 45: 557.
- ↑ Plotsky, P.M.; Sapolsky, Otto S., RM. (1986) "Inhibition of immunoreactive corticotropin-releasing factor secretion into the hypophysial portal circulation by delayed glucocorticoid feedback." Endocrinology 119: 1126,.
- ↑ Besedovsky, H.O.; Del Rey, A.; Sorkin, E. (1984) "Integration of Activated Immune Cell Products in Immune Endocrine Feedback Circuits." p. 200 in Leukocytes and Host Defense Vol. 5 [Oppenheim, J.J.; Jacobs, D.M., eds]. Alan R. Liss, New York,.
- ↑ Fairchild, S.L.; Shannon, K.; Kwan, E.; Mishell, R.I. (1984) "T-Cells Derived Glucocorticosteroid Lymphocytes and a T-Cell Hybridoma." Journal of Immunology 132: 821,.
- ↑ Dvorak, M.; "Plasma 17-Hydroxycorticosteroid Levels in Healthy and Diarrheic Calves." British Veterinarian Journal 127: 372, 1971.
- ↑ Besedovsky, H.O.; Del Rey, A.; Sorkin, E. (1984) "Integration of Activated Immune Cell Products in Immune Endocrine Feedback Circuits." p. 200 in Leukocytes and Host Defense Vol. 5 [Oppenheim, J.J.; Jacobs, D.M., eds]. Alan R. Liss, New York,.
- ↑ Fairchild, S.L.; Shannon, K.; Kwan, E.; Mishell, R.I. (1984) "T-Cells Derived Glucocorticosteroid Lymphocytes and a T-Cell Hybridoma." Journal of Immunology 132: 821,.
- ↑ Stith RD McCallum RE (1986) “General effect of endotoxin on glucocorticoid receptors in mammalian tissues. “ Circ. Shock 18(4); 301-309.
- ↑ Mikosha, A.S.; Pushkarov, I.S.; Chelnakova, I.S.; Remennikov, G.Y.A. (1991) “Potassium Aided Regulation of Hormone Biosynthesis in Adrenals of Guinea Pigs Under Action of Dihydropyridines: Possible Mechanisms of Changes in Steroidogenesis Induced by 1,4, Dihydropyridines in Dispersed Adrenocorticytes.” Fiziol. [Kiev] 37: 60,.
- ↑ Mendelsohn, F.A.; Mackie, C. (1975) “Relation of Intracellular K+ and Steroidogenesis in Isolated Adrenal Zona Glomerulosa and Fasciculata Cells.” Clinical Sci. Mol. Medical 49: 13,
- ↑ Ueda Y, Honda M, Tsuchiya M, Watanabe H, Izumi Y, Shiratsuchi T, Inoue T, Hatano M. (1982) “Response of plasma ACTH and adrenocortical hormones to potassium loading in essential hypertension.” Jpn Circ J. Apr;46(4):317-22.
- ↑ Bauman K Muller J 1972 “Effect of potassium on the final status of aldosterone biosynthesis in the rat. I 18-hydroxylation and 18hydroxy dehydrogenation. II beta-hydroxylation.” Acta Endocrin. Copenh. 69; I 701-717, II 718-730.
- ↑ LaCelle PL et al (1964) “An investigation of total body potassium in patients with rheumatoid arthritis.” Proceedings of the Annual Meeting of the American Rheumatism Association, Arthritis and Rheumatism 7; 321.
External links
|}
Target-derived NGF, BDNF, NT-3
|}
Template:Antidiarrheals, intestinal anti-inflammatory/anti-infective agents
Template:Otologicals
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |
