| Pyrimidine | |
|---|---|
| Chemical name | Pyrimidine |
| Chemical formula | C4H4N2 |
| Molecular mass | 80.09 g/mol |
| CAS number | [289-95-2] |
| Density | 1.016 g/cm3 |
| Melting point | 20–22 °C |
| Boiling point | 123–124 °C |
| SMILES | C1=NC=NC=C1 |
| Disclaimer and references | |
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring. It is isomeric with two other forms of diazine.
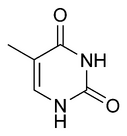
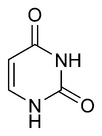
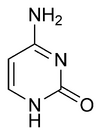
Three nucleobases found in nucleic acids, namely cytosine, thymine, and uracil, are pyrimidine derivatives. In DNA and RNA, these bases form hydrogen bonds with their complementary purines.
purine pyrimidine A T G C
In RNA, the complement of A is U instead of T:
purine pyrimidine A U G C
These hydrogen bonding modes are for classical Watson-Crick base pairing. Other hydrogen bonding modes ("wobble pairings") are available in both DNA and RNA, although the additional 2'-hydroxyl group of RNA expands the configurations through which RNA can form hydrogen bonds.
|
|
|
| Thymine | Uracil | Cytosine |
Pyrimidine biosynthesis[]
New synthesis of pyrimidine[]
Unlike purines, pyrimidines are assembled before being attached to 5-phosphoribosyl-1-pyrophosphate (PRPP). The first step begins with formation of carbamoyl phosphate by carbamoyl phosphate synthetase II.This is the regulated step in the pyrimidine biosynthesis. The second major step is the creation carbamoyl aspartic acid formed by aspartic transcarbamolyase (aspartate carbamoyl transferase).The next reaction involves dehydration of the acid catalysed by the enzyme dihhydroorotase to form hydroorotate. Dihydroorotate then enters the mitochondria where it is oxidised through removal of hydrogens to form orotate. This is the only mitochondrial step in nucleotide rings biosynthesis. The enzyme involved is dihydroorotate dehydrogenase (the only mitochondrial enzyme). Once orotate is eventually formed, it is combined with PRPP to form orotidine 5' monophosphate OMP which is decarboxylated in a reaction catalysed by OMP decarboxylase to form uridine 5' monophosphate. UMP is then converted to UDP catalysed by nucleotide diphosphokinase which is further phosphorylated to UTP by CTp synthase. This later reaction eventually leads to the formation of cytidine 5'triphosphate and glutamine is utlized.
Pyrmidine catabolism[]
Pyrimidines are ultimately catabolized (degraded) to CO2, H2O, and urea. Cytosine can be broken down to uracil which can be further broken down to N-carbamoyl-β-alanine. Thymine is broken down into β-aminoisobutyrate which can be further broken down into intermediates eventually leading into the citric acid cycle. β-aminoisobutyrate acts as a rough indicator for rate of DNA turnover.
See also[]
- Pyrazine, an analog with the nitrogen atoms in positions 1 and 4.
- Pyridazine, an analog with the nitrogen atoms in positions 1 and 2.
- Simple aromatic rings
External links[]
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - cDNA - snRNA - snoRNA - mtDNA - Oligonucleotide
|