Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
|
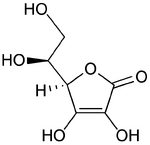
Ascorbic acid chemical structure | |
| 2-oxo-L-threo-hexono-1,4- lactone-2,3-enediol or (R)-3,4-dihydroxy-5-((S)- 1,2-dihydroxyethyl)furan-2(5H)-one IUPAC name | |
| CAS number 50-81-7 |
ATC code |
| PubChem 644104 |
DrugBank [1] |
| Chemical formula | |
| Molecular weight | 176.13 grams per mol |
| Bioavailability | rapid & complete |
| Metabolism | |
| Elimination half-life | 30 minutes |
| Excretion | renal |
| Pregnancy category | A |
| Legal status | general public availability |
| Routes of administration | oral |
This is a background article. See Psychological aspects of vitamin C
Vitamin C or L-ascorbic acid is an essential nutrient required in small amounts in order to allow a range of essential metabolic reactions in animals and plants. Vitamin C is widely known as the vitamin that prevents scurvy in humans.[1][2] The joint US-Canadian Dietary Reference Intake recommends 90 milligrams per day and no more than 2 grams per day (2000 milligrams per day),[3] although the amount that humans require for optimum health is a matter of heated debate.
Chemically, ascorbic acid exists in two forms: the active reduced form is ascorbic acid, while the oxidized form is dehydroascorbic acid. Dehydroscorbic acid present in the diet can be reduced to the active form in the body by enzymes and glutathione.[4] Ascorbic acid is an antioxidant and protects the body against oxidative stress,[5] as well as being needed as a coenzyme in some enzymatic reactions. The article on ascorbic acid contains further information on its chemical properties. This article describes its biological functions, discovery and the continuing scientific debate on how it is used by society, including its widespread application in doses larger than the officially recommended upper limit.
Biological significance[]
- Main article: ascorbic acid
Vitamin C is a weak sugar acid, and is a carbon based compound of six carbon atoms structurally related to glucose. Vitamin C is the L-enantiomer of ascorbic acid. The opposite D-enantiomer shows no biological activity. Both are mirror image forms of the same molecular structure. L-ascorbic acid exists as two inter-convertible compounds: L-ascorbic acid, which is a strong reducing agent, and its oxidised derivative, L-dehydroascorbic acid.[6]
The active part of the substance is the ascorbate ion, which occurs either attached to a hydrogen ion, forming ascorbic acid, or a metal ion, forming a mineral ascorbate. Some commercial vitamin C supplements contain a mix of ascorbic acid and mineral ascorbates.[6]
Function[]
- Vitamin C is a coenzyme, required for synthesis of:
- collagen, which is used in the connective tissue (participating in the hydroxylation). These fibers are ubiquitous throughout the body, providing firm but flexible structure. Some tissues have a greater percentage of collagen, especially: skin, mucous membranes, teeth and bones.
- dopamine, noradrenaline and adrenaline, which are used in the nervous system or in the adrenal glands.
- carnitine, which is used in the transfer of energy to the mitochondria.
- Vitamin C is an antioxidant, acting to lessen oxidative stress, and acts as a substrate for ascorbate peroxidase.
Biological tissues with over 100 times the level in blood plasma of vitamin C are the adrenal glands, pituitary, thymus, corpus luteum, and retina. Those with 10 to 50 times the concentration present in blood plasma include the brain, spleen, lung, testicle, lymph nodes, liver, thyroid, small intestinal mucosa, leukocytes, pancreas, kidney and salivary glands.
Natural mode of synthesis[]
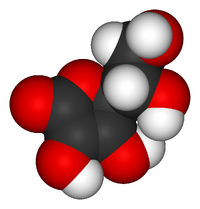
Model of a vitamin C molecule. Black is carbon, red is Oxygen and white is Hydrogen
The vast majority of animals and plants are able to synthesize their own vitamin C, achieved through a sequence of four enzyme driven steps, which convert glucose to vitamin C.[How to reference and link to summary or text] In reptiles and birds this is carried out in the kidneys, whereas in mammals and perching birds, it takes place in the liver.
Among the animals that have lost the ability to synthesis vitamin C are apes, guinea pigs, the red-vented bulbul, fruit-eating bats and a species of trout.[6] Most notably, along with the rest of the ape family in which we reside, humans have no capability to manufacture vitamin C. The cause of this phenomenon is that last enzyme in the synthesis process, L-gulonolactone oxidase, cannot be made by the listed animals because the gene for this enzyme is defective (named Pseudogene ΨGULO). The mutation has not been lethal because vitamin C is so prevalent in their food sources, with many of these species' natural diets consisting largely of fruit.
Some physicians and researchers, including chemist Linus Pauling have hypothesised that if the diets of the species who lost the ability to produce their own vitamin C were supplemented with an amount of the nutrient proportional to the amount produced in animal species that do synthesize their own vitamin C, better health would result. Most simians (higher primates who cannot produce vitamin C) consume the vitamin in amounts 10 to 20 times higher than that recommended by governments for humans (Milton, 1999). It has been noted that the loss of the ability to synthesize ascorbate strikingly parallels the evolutionary loss of the ability to break down uric acid. Uric acid and ascorbate are both strong reducing agents. This has led to the suggestion[7] that in higher primates, uric acid has taken over some of the functions of ascorbate. Ascorbic acid can be oxidised (broken down) in the human body by the enzyme ascorbic acid oxidase.
An adult goat, a typical example of a vitamin C-producing animal, who possesses all the necessary genes will manufacture more than 13,000 mg of vitamin C per day in normal health and as much as 100,000 mg daily when faced with life-threatening disease, trauma or stress.[8] Trauma or injury has also been demonstrated to use up large quantities of vitamin C in humans.[9]
Some microorganisms such as the yeast Saccharomyces cerevisiae have been shown to be able to synthesize vitamin C from simple sugars.[10][11]
Deficiency disease[]
Scurvy (a form of avitaminosis) results from lack of vitamin C, as an effect of its requirement for correct collagen synthesis. Scurvy leads to the formation of liver spots on the skin, spongy gums, and bleeding from all mucous membranes. The spots are most abundant on the thighs and legs, and a person with the ailment looks pale, feels depressed, and is partially immobilized. In advanced scurvy there are open, suppurating wounds and loss of teeth, and eventually, death.
Historically, scurvy was common among those with poor access to fresh fruit and vegetables, such as sailors, pirates and others who were on ships that were out to sea longer than perishable fruits and vegetables could be stored, as well as isolated soldiers. The earliest documented case was described by Hippocrates around the year 400 BC.
The first attempt to give scientific basis for the cause of scurvy was by a ship's surgeon in the British Royal Navy, James Lind. While at sea in May 1747, Lind provided some crew members with two oranges and one lemon per day, in addition to normal rations, while others continued on cider, vinegar, sulfuric acid or seawater, along with their normal rations. The results conclusively showed that something in the citrus fruits prevented the disease, a property later described as "antiscorbutic". Vitamin C was isolated in the 1920s by Albert Szent-Györgyi and was shown to be ascorbic acid.
No bodily organ stores vitamin C,[How to reference and link to summary or text] and so the body soon depletes itself if fresh supplies are not consumed through the digestive system.
Daily dosage requirements[]
There is continuing debate within the scientific community over the best dose schedule (the amount and frequency of intake) of vitamin C for maintaining optimal health in humans.[12] It is generally agreed that balanced diet without supplementation contains enough vitamin C to prevent acute scurvy in an average healthy adult (those who are pregnant, smoke tobacco, or are under stress require slightly more).[3]
Vitamin C is recognized to be one of the least toxic substances known to medicine,[3] with the LD50 being 11,900 milligrams per kilogram.[13][14] High doses (thousands of milligrams) may result in diarrhoea, which is harmless if the dose is reduced immediately. Some researchers[15] claim the onset of diarrhoea to be an indication of where the body’s true vitamin C requirement lies. Both Cathcart[15] and Cameron have demonstrated that very sick patients with cancer or influenza do not display any evidence of diarrhoea at all until ascorbate intake reaches levels as high as 200 grams (half a pound).
Government recommended intake levels[]
| United States vitamin C recommendations[3] | |
|---|---|
| Recommended Dietary Allowance (adult male) | 90 mg per day |
| Recommended Dietary Allowance (adult female) | 75 mg per day |
| Tolerable Upper Intake Level (adult male) | 2000 mg per day |
| Tolerable Upper Intake Level (adult female) | 2000 mg per day |
Recommendations for vitamin C intake have been set by various national agencies:
- 40 milligrams per day — the United Kingdom's Food Standards Agency[1]
- 45 milligrams per day — the World Health Organization[16]
- 60-95 milligrams per day — United States' National Academy of Sciences[3]
The United States defined Tolerable Upper Intake Level for a 25-year old male is 2000 milligrams per day.
Independent recommended intake levels[]
Some independent researchers have calculated the amount needed for an adult human to achieve similar blood serum levels as vitamin C synthesising mammals as follows:
- 400 milligrams per day — the Linus Pauling Institute and the US National Institutes of Health
- 500 milligrams per 12 hours — Professor Roc Ordman, from research into biological free radicals[17]
- 3,000 milligrams per day (or more during illness or pregnancy, sometimes up to 300,000 mg) — the Vitamin C Foundation[18]
- 6,000–12,000 milligrams per day — Thomas Levy, Colorado Integrative Medical Centre
- 6,000–18,000 milligrams per day — Linus Pauling's person use[19]
- 3,000–200,000 milligrams per day — Robert Cathcart's protocol known as a "vitamin C flush" wherein escalating doses of vitamin C are given until diarrhoea develops, then choosing the highest dose that does not cause diarrhoea (the bowel tolerance threshold)[15]
Testing for ascorbate levels in the body[]
Simple tests exist for measurng the levels of vitamin C in the urine and in serum or blood plasma. However these do not accurately reflect actual tissue ascorbate levels.[How to reference and link to summary or text] Reverse phase high performance liquid chromatography is used for determining the storage levels of vitamin C within lymphocytes and tissue.
It has been observed that while serum or blood plasma levels follow the circadian rhythm or short term dietary changes those within tissues themselves are more stable and give a better view of the availability of ascorbate within the organism. However, very few hospital laboratories are adequately equipped and trained to carry out such detailed analyses, and require samples to be analyzed in specialized laboratories.[20][21]
Vitamin C as a macronutrient[]

Linus Pauling's popular and influential book How to Live Longer and Feel Better, first published in 1986, advocated very high doses of vitamin C.
- Main article: Vitamin C megadosage
There is a strong advocacy movement for large doses of vitamin C, promoting a great deal of added benefits, despite not all benefits being agreed upon by the medical community. Many pro-vitamin C organizations promote usage levels well beyond the current Dietary Reference Intake. The movement is fronted by scientists and doctors such as Robert Cathcart, Steve Hickey, Irwin Stone and the twice Nobel Prize laureate Linus Pauling and the more controversial Matthias Rath. There is an extensive and growing literature critical of governmental agency dose recommendations.[12][22]
Hickey believes that humans carry a mutated and ineffective form of the gene required by all mammals for manufacturing the fourth of the four enzymes that manufacture vitamin C. The gene, Pseudogene ΨGULO, is thought to have lost its function millions of years ago.[23] In humans the three surviving enzymes continue to produce the precursors to vitamin C, but the process is incomplete and the body then disassembles them. Stone and Pauling later hypothesized that our evolutionary ancestors could once manufacture this substance in estimated quantities 3,000–4,000 mg daily. If true, this would mean that vitamin C was misnamed as a vitamin and is in fact a vital macronutrient like protein or carbohydrate.[24]
The biological halflife for vitamin C is fairly short, about 30 minutes in blood plasma, a fact which high dose advocates say that mainstream researchers have failed to take into account. Researchers at the National Institutes of Health decided upon the current RDA based upon tests conducted 12 hours (24 half lives) after consumption. Hickey, on this matter, says "To be blunt, the NIH gave a dose of vitamin C, waited until it had been excreted, and then measured blood levels."[25]
Possible other vitamin C deficiencies[]
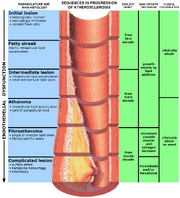
Atherosclerosis has been hypothesised to be a vitamin C deficiency disease
The established RDA has been criticised by Pauling to be one that will prevent acute scurvy, and is not necessarily the dosage for optimal health.[How to reference and link to summary or text]
The controversial Matthias Rath hypothesised that during the ice age, when vitamin C was scarce, natural selection favoured human individuals who could repair arteries with a layer of cholesterol. He suggests that although eventually harmful, cholesterol lining of artery walls would be beneficial in that it would keep the individual alive until access to vitamin C allowed arterial damage to be repaired. If this is true, atherosclerosis is in fact a vitamin C deficiency disease. As atherosclerosis is the main cause of ischaemic heart disease, which in turn is the leading cause of death in developed countries,[26] this would have a profound effect on western medicine.
Therapeutic applications of high doses[]
Since its discovery vitamin C has been considered a "universal panacea" by some, although this led to suspicions of it being overhyped by others.[27] It has been hypothosised that the vitamin can prevent and cure a wide range of common and/or lethal diseases, ranging from the common cold and cataracts to controversial statements involving it being a cure for AIDS[28] SARS and bird flu.[29][30] There have even been suggestions that vitamin C can cure lead poisoning and autism.
Possible adverse effects[]
While being harmless in most typical quantities, as with all substances to which the human body is exposed, vitamin C can still cause harm under certain conditions. In the medical community, these are known as contraindications.
- As vitamin C enhances iron absorption, iron poisoning can become an issue to people with rare iron overload disorders, such as haemochromatosis.
- A genetic condition that results in inadequate levels of the enzyme glucose-6-phosphate dehydrogenase (G6PD), can cause sufferers to develop hemolytic anemia after ingesting specific oxidizing substances, such as very large dosages of vitamin C. However, there is a test available for G6PD deficiency,[31] and it has been proposed that high doses of Vitamin E may protect against this problem.
List of side-effects[]
- Relatively large doses of vitamin C may cause indigestion, particularly when taken on an empty stomach. This unpleasant but harmless side-effect can be avoided by taking the vitamin along with meals or by offsetting its acidity by taking an antacid such as baking soda or calcium carbonate.
- When taken in huge doses, vitamin C causes diarrhoea. The minimum dose that brings about this effect varies on the individual. Robert Cathcart has called this limit the "bowel tolerance threshold" and observed that it is higher in people with serious illness than those in good health.[15] It ranges from 5 to 25 grams per day in healthy individuals to 300 grams per day in those that are severely ill. Diarrhoea is not harmful, as long as dose is reduced quickly.
Chance of overdose[]
As discussed previously, vitamin C exhibits remarkably low toxicity. The LD50 (the dose that will kill 50% of a population) is generally accepted to be 11900 milligrams per kilogram.[32] This means that for a 60 kilo (132 pound) human, one would need to administer 714,000 mg (714 g or 1.6 pounds) of vitamin C in order to to stand a 50% chance of killing the person. However, vitamin C cannot result in death when taken orally as large amounts of the vitamin cause diarrhea and are not absorbed.[33] An extremely large amount of vitamin C would need to be rapidly injected in order to stand any chance of killing a person. Supposedly, Robert Cathcart has used intravenous doses of up to 250 grams with no adverse effects.[34] The United States Council for Responsible Nutrition has set an Upper Level of 2 grams, based on transient diarrhoea. Their publication on vitamin C safety notes that[33]
| “ | Very large doses of vitamin C have been taken daily over the course of many years, and only minor undesirable effects have been attributed with any certainty to the vitamin’s use... Clearly, vitamin C has a low order of toxicity. | ” |
Natural and artificial dietary sources[]
Vitamin C is obtained through the diet by the vast majority of the world's population. The richest natural sources are fruits and vegetables, and of those, the camu camu fruit and the billygoat plum contain the highest concentration of the vitamin. It is also present in some cuts of meat, especially liver. Vitamin C is the most widely taken nutritional supplement and is available in a variety of forms, including tablets, drink mixes, crystals in capsules or naked crystals.
Plant sources[]

Rose hips are a particularly rich source of vitamin C
The amount of vitamin C in foods of plant origin depends on:
- the precise variety of the plant,
- the soil condition
- the climate in which it grew,
- the length of time since it was picked,
- the storage conditions,
- the method of preparation. Cooking in particular is often said to destroy vitamin C — but see the section on Food preparation.
The following table is approximate and shows the relative abundance in different raw plant sources. The amount is given in milligrams per 100 grams of fruit or vegetable:
| Plant source | Amount (mg/100 g) |
|---|---|
| Billy Goat plum | 3150 |
| Camu Camu | 2800 |
| Wolfberry | 2500 |
| Rose hip | 2000 |
| Acerola | 1600 |
| Amla | 720 |
| Jujube | 500 |
| Baobab | 400 |
| Blackcurrant | 200 |
| Red pepper | 190 |
| Parsley | 130 |
| Seabuckthorn | 120 |
| Guava | 100 |
| Kiwifruit | 90 |
| Broccoli | 90 |
| Loganberry | 80 |
| Redcurrant | 80 |
| Brussels sprouts | 80 |
| Lychee | 70 |
| Cloudberry | 60 |
| Persimmon | 60 |
| Plant source | Amount (mg/100 g) |
|---|---|
| Papaya | 60 |
| Strawberry | 60 |
| Orange | 50 |
| Lemon | 40 |
| Melon, cantaloupe | 40 |
| Cauliflower | 40 |
| Grapefruit | 30 |
| Raspberry | 30 |
| Tangerine | 30 |
| Mandarin orange | 30 |
| Passion fruit | 30 |
| Spinach | 30 |
| Cabbage raw green | 30 |
| Lime | 20 |
| Mango | 20 |
| Potato | 20 |
| Melon, honeydew | 20 |
| Mango | 16 |
| Tomato | 10 |
| Blueberry | 10 |
| Pineapple | 10 |
| Plant source | Amount (mg/100 g) |
|---|---|
| Pawpaw | 10 |
| Grape | 10 |
| Apricot | 10 |
| Plum | 10 |
| Watermelon | 10 |
| Banana | 9 |
| Carrot | 9 |
| Avocado | 8 |
| Crabapple | 8 |
| Peach | 7 |
| Apple | 6 |
| Blackberry | 6 |
| Beetroot | 5 |
| Pear | 4 |
| Lettuce | 4 |
| Cucumber | 3 |
| Eggplant | 2 |
| Fig | 2 |
| Bilberry | 1 |
| Horned melon | 0.5 |
| Medlar | 0.3 |
Animal sources[]

Goats, like almost all animals, make their own vitamin C. An adult goat will manufacture more than 13,000 mg of vitamin C per day in normal health and as much as 100,000 mg daily when faced with life-threatening disease, trauma or stress.
As noted previously, the overwhelming majority of species of animals and plants synthesise their own vitamin C, making animal products, such as meat, a source of dietary vitamin C.
Vitamin C is present in mother's milk and in less amounts in raw cow's milk, with pasteurized milk containing only trace amounts.[35]
The following table shows the relative abundance of vitamin C in various foods of animal origin, given in milligram of vitamin C per 100 grams of food:
| Food | Amount (mg/100 g) |
|---|---|
| Calf liver (raw) | 36 |
| Beef liver (raw) | 31 |
| Oysters (raw) | 30 |
| Cod roe (fried) | 26 |
| Pork liver (raw) | 23 |
| Lamb brain (boiled) | 17 |
| Chicken liver (fried) | 13 |
| Lamb liver (fried) | 12 |
| Lamb heart (roast) | 11 |
| Food | Amount (mg/100 g) |
|---|---|
| Lamb tongue (stewed) | 6 |
| Human milk (fresh) | 4 |
| Goat milk (fresh) | 2 |
| Cow milk (fresh) | 2 |
| Beef steak (fried) | 0 |
| Hen's egg (raw) | 0 |
| Pork bacon (fried) | 0 |
| Calf veal cutlet (fried) | 0 |
| Chicken leg (roast) | 0 |
Food preparation[]
Vitamin C chemically decomposes under certain conditions, many of which may occur during the cooking of food. Normally, boiling water at 100°C is not hot enough to cause any significant destruction of the nutrient, which only decomposes at 190°C, despite popular opinion. However, pressure cooking, roasting, frying and grilling food is more likely to reach the decomposition temperature of Vitamin C. Longer cooking times also add to this effect, as will copper food vessels, which catalyse the decomposition.[13]
Another source of the removal of vitamin C from food is leaching, where the water-soluble vitamin dissolves into the cooking water, which is later poured away and not eaten. However, vitamin C doesn't leach in all vegetables at the same rate; research shows broccoli seems to retain more than any other.[36]
Research has shown that fresh-cut fruit won't lose significant nutrients when stored in the refrigerator for a few days.[37]
Vitamin C supplements[]
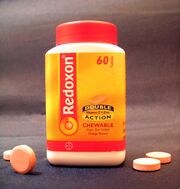
Vitamin C is widely available in the form of tablets and powders. The Redoxon brand, launched in 1934 by Hoffmann-La Roche, was the first mass-produce synthetic vitamin C.
Vitamin C is the most widely taken dietary supplement.[38] It is available in many forms including caplets, tablets, capsules, drink mix packets, in multi-vitamin formulations, in multiple anti-oxidant formulations, as chemically pure crystalline powder, time release versions, and also including bioflavonoids such as quercetin, hesperidin and rutin. Tablet and capsule sizes range from 25 mg to 1500 mg. Vitamin C (as ascorbic acid) crystals are typically available in bottles containing 300 g to 1 kg of powder (a teaspoon of vitamin C crystals equals 5,000 mg). In supplements, Vitamin C most often comes in the form of various mineral ascorbates, as they are easier to absorb, more easily tolerated and provide a source of several dietary minerals.
Artificial modes of synthesis[]
Vitamin C is produced from glucose by two main routes. The Reichstein process, developed in the 1930s, uses a single pre-fermentation followed by a purely chemical route. The modern two-step fermentation process, originally developed in China in the 1960s, uses additional fermentation to replace part of the later chemical stages. Both processes yield approximately 60% vitamin C from the glucose feed.[39]
Research is underway at the Scottish Crop Research Institute in the interest of creating a strain of yeast that can synthesise vitamin C in a single fermentation step from galactose, a technology expected to reduce manufacturing costs considerably.[10]
World production of synthesised vitamin C is currently estimated at approximately 110,000 tonnes annually. Main producers today are BASF/Takeda, DSM, Merck and the China Pharmaceutical Group Ltd. of the People's Republic of China. China is slowly becoming the major world supplier as its prices undercut those of the US and European manufacturers.[40]
History[]

James Lind, a British Royal Navy surgeon who, in 1774, identified that a quality in fruit prevented the disease of scurvy in what was the first recorded controlled experiment.
The need to include fresh plant food or raw animal flesh in the diet to prevent disease was known from ancient times. Native peoples living in marginal areas incorporated this into their medicinal lore. For example, infusions of spruce needles were used in the temperate zones, or the leaves from species of drought-resistant trees in desert areas. In 1536, the French explorer Jacques Cartier, exploring the St. Lawrence River, used the local natives' knowledge to save his men who were dying of scurvy. He boiled the needles of the arbor vitae tree to make a tea that was later shown to contain 50 mg of vitamin C per 100 grams.[41][42]
Through history the benefit of plant food for the survival of long sea voyages had been occasionally recommended by authorities. John Woodall, the first appointed surgeon to the British East India Company, recommended the use of lemon juice as a preventive and cure in his book "The Surgeon's Mate" of 1617. The Dutch writer, Johann Bachstrom, in 1734, gave the firm opinion that "scurvy is solely owing to a total abstinence from fresh vegetable food, and greens; which is alone the primary cause of the disease."

Citrus fruits were one of the first sources of vitamin C available to ship's surgeons.
The first attempt to give scientific basis for the cause of scurvy was by a ship's surgeon in the British Royal Navy, James Lind. While at sea in May 1747, Lind provided some crew members with two oranges and one lemon per day, in addition to normal rations, while others continued on cider, vinegar or seawater, along with their normal rations. In the history of science this is considered to be the first example of a controlled experiment comparing results on two populations of a factor applied to one group only with all other factors the same. The results conclusively showed that citrus fruits prevented the disease. Lind wrote up his work and published it in 1753, in Treatise on the Scurvy.
Lind's work was slow to be noticed, partly because he gave conflicting evidence within the book and partly because the British admiralty saw care for the well-being of ships' crew as a sign of weakness. Also, fresh fruit was very expensive to keep on board, whereas boiling it down to juice allowed easy storage but destroyed the vitamin (especially if boiled in copper kettles[13]). Ship captains assumed wrongly that it didn't work, because the juice failed to cure scurvy.
It was 1795 before the British navy adopted lemons or lime as standard issue at sea. Limes were more popular as they could be found in British West Indian Colonies, unlike lemons which weren't found in British Dominions, and were therefore more expensive. This practice led to the use of the nickname "limey" to refer to the British. Captain James Cook had previously demonstrated and proven the principle of the advantages of fresh and preserved foods, such as sauerkraut, by taking his crews to the Hawaiian Islands and beyond without losing any of his men to scurvy. For this otherwise unheard of feat, the British Admiralty awarded him a medal.
The name "antiscorbutic" was used in the eighteenth and nineteenth centuries as general term for those foods known to prevent scurvy, even though there was no understanding of the reason for this. These foods included but were not limited to: lemons, limes, and oranges; sauerkraut, cabbage, malt, and portable soup.
In 1907, Axel Holst and Theodor Frølich, two Norwegian physicians studying beriberi contracted aboard ship's crews in the Norwegian Fishing Fleet, wanted a small test mammal to substitute for the pigeons they used. They fed guinea pigs their test diet, which had earlier produced beriberi in their pigeons, and were surprised when scurvy resulted instead. Until that time scurvy had not been observed in any organism apart from humans, and had been considered an exclusively human disease.
Discovery of ascorbic acid[]

Albert Szent-Györgyi, pictured here in 1948, was awarded the 1937 Nobel Prize in Medicine for the discovery of vitamin C
In 1912, the Polish-American biochemist Casimir Funk, while researching deficiency diseases, developed the concept of vitamins to refer to the nutrients which are essential to health. Then, from 1928 to 1933, the Hungarian research team of Joseph L Svirbely and Albert Szent-Györgyi and, independently, the American Charles Glen King, first isolated vitamin C and showed it to be ascorbic acid. Although Szent-Györgyi was awarded the 1937 Nobel Prize in Medicine, many feel King is as responsible for its development.[43]
In 1928 the Arctic anthropologist Vilhjalmur Stefansson attempted to prove his theory of how the Eskimos are able to avoid scurvy with almost no plant food in their diet, despite the disease striking European Arctic explorers living on similar high-meat diets. Stefansson theorised that the natives get their vitamin C from fresh meat that is minimally cooked. Starting in February 1928, for one year he and a colleague lived on an exclusively minimally-cooked meat diet while under medical supervision; they remained healthy.
Between 1933 and 1934, the British chemists Sir Walter Norman Haworth and Sir Edmund Hirst and, independently, the Polish chemist Tadeus Reichstein, succeeded in synthesizing the vitamin, the first to be artificially produced. This made possible the cheap mass production of vitamin C. Only Haworth was awarded the 1937 Nobel Prize in Chemistry for this work, but the process for vitamin C retained Reichstein's name.
In 1934 the Hoffmann–La Roche became the first pharmaceutical company to mass-produce synthetic vitamin C, under the brand name of Redoxon.
In 1959 the American J.J. Burns showed that the reason some mammals were susceptible to scurvy was the inability of their liver to produce the active enzyme L-gulonolactone oxidase, which is the last of the chain of four enzymes which synthesize vitamin C.[44][45] American biochemist Irwin Stone was the first to exploit vitamin C for its food preservative properties. He later developed the theory that humans possess a mutated form of the L-gulonolactone oxidase coding gene.
See also[]
.
- Ascorbic acid — for the chemistry of vitamin C
- Ascorbyl palmitate — a fat-soluble ester of vitamin C
- Ascorbyl stearate — a fat-soluble ester of vitamin C
- Dehydroascorbic acid — an oxidized form of vitamin C
- Erythorbic acid — a stereoisomer of vitamin C
- Erythorbin acid — a stereoisomer of vitamin C
- Mineral ascorbates — salts of vitamin C
- Uric acid — the loss of the ability to process uric acid in higher primates parallels the loss of the ability to synthesize vitamin C
- Vitamin C megadosage — for the arguments for and against the controversial theory of vitamin C being a "universal panacea"
- Dynamic Flow — for the arguments for and against the controversial theory of vitamin C being a "universal panacea"
- General
- Antioxidant — chemicals that slow or prevent oxidation reactions, vitamin C being one
- Coenzyme — non-protein molecules that carry chemical groups between enzymes, vitamin C being one
- Micronutrient — essential nutrients needed for life in small quantities
- Macronutrient — essential nutrients needed for life in large quantities
- Megavitamin therapy — the use of large amounts of vitamins, often many times greater than the recommended dietary allowance, in the prevention and treatment of diseases
- Orthomolecular medicine — the correction of abnormal levels of any molecules in the body
- Schizophrenia - Adrenochrome hypothesis mega doses may treat schizophrenia?
- Vitamin — nutrients required in very small amounts for essential metabolic reactions in the body
References[]
- ↑ 1.0 1.1 Vitamin C. Food Standards Agency (UK). URL accessed on 2007-02-19.
- ↑ Vitamin C (Ascorbic Acid). University of Maryland Medical Center. URL accessed on 2007-02-19.
- ↑ 3.0 3.1 3.2 3.3 3.4 US Recommended Dietary Allowance (RDA). URL accessed on 2007-02-19.
- ↑ Meister A (1994). Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem 269 (13): 9397-400.
- ↑ Padayatty S, Katz A, Wang Y, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta S, Levine M (2003). Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22 (1): 18-35.
- ↑ 6.0 6.1 6.2 Vitamin C – Risk Assessment. UK Food Standards Agency. URL accessed on 2007-02-19.
- ↑ Proctor, P. "Similar Functions of Uric Acid and Ascorbate in Man", Nature, 228(5274), 868. DOI:10.1038/228868a0
- ↑ M. Ellert. Vitamins and Minerals. Southern Illinois University, School of Medicine. URL accessed on 2007-02-28.
- ↑ C. Long, et al. Ascorbic acid dynamics in the seriously ill and injured. Journal of Surgical Research, 109(2), 144–148. DOI:10.1016/S0022-4804(02)00083-5 - "Our results show that plasma ascorbic acid levels following trauma and during infection are extremely low and are not normalized with 300 or even 1000 mg/day supplemented TPN. " Accessed January 2007
- ↑ 10.0 10.1 R.D. Hancock & R. Viola. Ascorbic acid biosynthesis in higher plants and micro-organisms. Scottish Crop Research Institute. URL accessed on 2007-02-20.
- ↑ Hancock RD, Galpin JR, Viola R.. Biosynthesis of L-ascorbic acid (vitamin C) by Saccharomyces cerevisiae. FEMS Microbiol Lett. 186 (2): 245-50.
- ↑ 12.0 12.1 Linus Pauling Vindicated; Researchers Claim RDA For Vitamin C is Flawed. PR Newswire. URL accessed on 2007-02-20.
- ↑ 13.0 13.1 13.2 Safety data for ascorbic acid. Oxford University. URL accessed on 2007-02-20.
- ↑ Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. World Health Organization. URL accessed on 2007-02-20.
- ↑ 15.0 15.1 15.2 15.3 Robert F. Cathcart III M.D.. Vitamin C, Titrating To Bowel Tolerance, Anascorbemia, and Acute Induced Scurvy. Orthomed. URL accessed on 2007-02-22.
- ↑ Vitamin and mineral requirements in human nutrition, 2nd edition. World Health Organization. URL accessed on 2007-02-20.
- ↑ Roc Ordman. The Scientific Basis Of The Vitamin C Dosage Of Nutrition Investigator. Beloit College. URL accessed on 2007-02-22.
- ↑ Vitamin C Foundation's RDA. URL accessed on 2007-02-12.
- ↑ Linus Pauling (1986). How to Live Longer and Feel Better, W. H. Freeman and Company.
- ↑ Emadi-Konjin P, Verjee Z, Levin A, Adeli K (2005). Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC).. Clin Biochem 38 (5): 450-6. PMID 15820776.
"Serum and plasma vitamin C measurements do not correlate well with tissue levels while lymphocyte vitamin C levels provide the most accurate assessment of the true status of vitamin C stores and are not affected acutely by circadian rhythm or dietary changes."
- ↑ Yamada H, Yamada K, Waki M, Umegaki K. (2004). Lymphocyte and Plasma Vitamin C Levels in Type 2 Diabetic Patients With and Without Diabetes Complications. Diabetes Care 27: 2491–2.
"The plasma concentration of vitamin C is considered to be strongly correlated with transient consumption of foods. The measurement of lymphocyte vitamin C might be expected to be a more reliable antioxidant biomarker than plasma vitamin C level. In this report, we demonstrated that the lymphocyte vitamin C level is significantly lower in type 2 diabetic patients, but we could not observe such an association in plasma vitamin C levels. In diabetes, therefore, the measurement of lymphocyte vitamin C might be expected to be a more reliable antioxidant biomarker than plasma vitamin C level."
- ↑ Robert Forman (1981). Medical Resistance To Innovation. Medical Hypotheses 7 (8): 1009-1017.
- ↑ (2004) Ascorbate: The Science of Vitamin C, Lulu Press, Inc..
- ↑ Irwin Stone (1972). The Healing Factor: Vitamin C Against Disease, Grosset and Dunlap.
- ↑ Bill Sardi. The Vitamin C Fanatics Were Right All Along. Knowledge of Health, Inc.. URL accessed on 2007-02-22.
- ↑ WHO World Health Report 2002
- ↑ Harri Hemilä. Do vitamins C and E affect respiratory infections?. University of Helsinki. URL accessed on 2007-02-21.
- ↑ Nigeria: Vitamin C Can Suppress HIV/Aids Virus. allAfrica.com. URL accessed on 2006-06-16.
- ↑ Sarah Boseley. Discredited doctor's 'cure' for Aids ignites life-and-death struggle in South Africa. The Guardian. URL accessed on 2007-02-21.
- ↑ Dr. Matthias Rath. Open letter from Dr. Matthias Rath MD to German Chancellor Merkel. Dr. Rath Health Foundation. URL accessed on 2007-02-21.
- ↑ Intravenous Ascorbate as a Chemotherapeutic and Biologic Response Modifying Agent. The Center For The Improvement Of Human Functioning International. URL accessed on 2007-02-19.
- ↑ Safety (MSDS) data for ascorbic acid. Oxford University. URL accessed on 2007-02-21.
- ↑ 33.0 33.1 John N. Hathcock. Water-Soluble Vitamins - Vitamin C. Council for Responsible Nutrition. URL accessed on 2007-02-21.
- ↑ ROBERT F. CATHCART III. Preparation of Sodium Ascorbate for IV and IM Use. orthomed.com. URL accessed on 2007-02-21.
- ↑ Stephanie Clark, Ph.D. Comparing Milk: Human, Cow, Goat & Commercial Infant Formula. Washington State University. URL accessed on 2007-02-28.
- ↑ Combs GF. The Vitamins, Fundamental Aspects in Nutrition and Health. 2nd ed. San Diego, CA: Academic Press, 2001:245–272
- ↑ Miranda Hitti. Fresh-Cut Fruit May Keep Its Vitamins. WebMD. URL accessed on 2007-02-25.
- ↑ The Diet Channel Vitamin C might be the most widely known and most popular vitamin purchased as a supplement.
- ↑ The production of vitamin C. Competition Commission. URL accessed on 2007-02-20.
- ↑ Dominique Patton. DSM makes last stand against Chinese vitamin C. nutraingredients. URL accessed on 2007-02-20.
- ↑ Jacques Cartier's Second Voyage - 1535 - Winter & Scurvy. URL accessed on 2007-02-25.
- ↑ Martini E. (June 2002). Jacques Cartier witnesses a treatment for scurvy. Vesalius.
- ↑ Pitt History. University of Pittsburgh. URL accessed on 2007-02-21.
- ↑ Burns, J. J., and Evans, C., J. Biol. Chem., 200, 125 (1953).
- ↑ Burns, J. J., Peyser, P., and Maltz, A., Science, 124, 1148 (1956).
- Levy Thomas (2002). Vitamin C, Infectious Diseases, and Toxins, Xlibris Corporation (Paperback). ISBN 1-4010-6963-0.(Note: Xlibris is a print on demand self-publishing house.)
- Dolske, M.C., et al. 1993. "A preliminary trial of ascorbic acid as a supplemental therapy for autism." Prog. Neuropsychopharmacol. Biol. Psychiatry, 17(5):765–774.
- Green, V.A., K.A. Pituch, J. Itchon, A. Choi, M. O'Reilly, J. Sigafoos, "Internet survey of treatments used by parents of children with autism," Res Dev Disabil, 2006, 27(1):70–84.
- Milton, K. (1999) "Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us?" Nutrition. 1999 Jun;15(6):488-98.
Further reading[]
- Cancer and Vitamin C, Ewan Cameron and Linus Pauling, Pauling Institute of Science and Medicine, 1979
- Life Extension: A Practical Scientific Approach (Part IV, Chapter 7: Vitamin C), Durk Pearson and Sandy Shaw, Warner Books, 1982
- Life Extension Revolution, Saul Kent, Morrow, 1980
- Mind Food and Smart Pills: How to Increase Your Intelligence and Prevent Brain Aging (Chapter 3: Vitamin C, The Champion Free Radical Scavenger), Ross Pelton, 1986
- Vitamin C and the Common Cold, Linus Pauling, 1970
- Vitamin C, the Common Cold, and the Flu, Linus Pauling, Freeman, 1976
- Vitamin C, Volumes I, II, III., Monograph by C.A.B Clemetson, 1989 CRC Press, Boca Raton, Florida, ISBN 0-8493-4841-2
External links[]
- Jane Higdon, "Vitamin C", Micronutrient Information Center, Linus Pauling Institute
- AscorbateWeb — a collection of twentieth century medical & scientific literature on vitamin C in the treatment and prevention of human disease
- Healing Thresholds — summaries of research on Vitamin C and other autism therapies
- U.S. Patent 5,278,189
— Prevention and treatment of occlusive cardiovascular disease with ascorbate and substances that inhibit the binding of lipoprotein (A), Inventors: Matthias W. Rath and Linus C. Pauling
- Natural food-Fruit Vitamin C Content
- United Kingdom Food Standards Agency on vitamin C
- Vitamin C in human health and disease is still a mystery? An overview — one of BioMed Central most popular pages
- Vitamin C May Not Have Much Effect on Colds — 200 mg per day has little effect on colds but a single dose of 8 grams does
- Vitamin C Requirements: Optimal Health Benefits vs Overdose — high dose advocacy site
- Ascorbate and cancer — discussion of both historical and current uses of Vitamin C in cancer treatment
- For Doctors: Preparation of Vitamin C IV's — by Andrew W. Saul, PhD.
- Information regarding treatment of the Bird Flu with massive doses of ascorbate. — by Robert Cathcart, M.D.
| Vitamins |
|---|
| All B vitamins | All D vitamins |
| Retinol (A) | Thiamine (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic acid (B5) | Pyridoxine (B6) | Biotin (B7) | Folic acid (B9) | Cyanocobalamin (B12) | Ascorbic acid (C) | Ergocalciferol (D2) | Cholecalciferol (D3) | Tocopherol (E) | Naphthoquinone (K) |
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |