Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Clinical: Approaches · Group therapy · Techniques · Types of problem · Areas of specialism · Taxonomies · Therapeutic issues · Modes of delivery · Model translation project · Personal experiences ·
| ICD-10 | E800-E802 | |
|---|---|---|
| ICD-9 | 277.1 | |
| OMIM | [1] | |
| DiseasesDB | [2] | |
| MedlinePlus | 001208 | |
| eMedicine | / | |
| MeSH | C17.800.849.617 | |
Porphyrias are a group of inherited or acquired disorders of certain enzymes in the heme biosynthetic pathway (also called porphyrin pathway). They are broadly classified as acute (hepatic) porphyrias and cutaneous (erythropoietic) porphyrias, based on the site of the overproduction and accumulation of the porphyrins (or their chemical precursors). They manifest with either skin problems or with neurological complications (or occasionally both). A clinically and histologically identical condition is called pseudoporphyria. Pseudoporphyria is characterized by normal serum and urine porphyrin levels.
The term derives from the Greek πορφύρα, porphura, meaning "purple pigment". The name is likely to have been a reference to the purple discolouration of feces and urine in patients during an attack.[1] Although original descriptions are attributed to Hippocrates, the disease was first explained biochemically by Dr Felix Hoppe-Seyler in 1874,[2] and acute porphyrias were described by the Dutch physician Prof B.J. Stokvis in 1889.[1][3]
Signs and symptoms[]
Acute porphyria[]
The acute, or hepatic, porphyrias primarily affect the nervous system, resulting in abdominal pain, vomiting, acute neuropathy, seizures and mental disturbances, including hallucinations, depression, anxiety and paranoia. Cardiac arrhythmias and tachycardia (fast heart rate) may develop as the autonomic nervous system is affected. Pain can be severe and can, in some cases, be both acute and chronic in nature. Constipation is frequently present, as the nervous system of the gut is affected, but diarrhea can also occur.
Given the many presentations and the relatively uncommon occurrence of porphyria the patient may initially be suspected to have other, unrelated conditions. For instance, the polyneuropathy of acute porphyria may be mistaken for Guillain-Barré syndrome, and porphyria testing is commonly recommended in those scenarios.[4] Systemic lupus erythematosus features photosensitivity, pain attacks and shares various other symptoms with porphyria.[5]
Not all porphyrias are genetic, and patients with liver disease who develop porphyria as a result of liver dysfunction may exhibit other signs of their condition, such as jaundice.
Patients with acute porphyria (PCT, AIP, HCP, VP) are at increased risk over their life for hepatocellular carcinoma (primary liver cancer) and may require monitoring. Other typical risk factors for liver cancer need not be present, such as hepatitis B or C, iron overload or alcoholic cirrhosis.
Cutaneous porphyria[]
The cutaneous, or erythropoietic, porphyrias primarily affect the skin, causing photosensitivity (photodermatitis), blisters, necrosis of the skin and gums, itching, and swelling, and increased hair growth on areas such as the forehead. Often there is no abdominal pain, distinguishing it from other porphyrias.
In some forms of porphyria, accumulated heme precursors excreted in the urine may cause various changes in color, after exposure to sunlight, to a dark reddish or dark brown color. Even a purple hue or red urine may be seen. Heme precursors may also accumulate in the teeth and fingernails, giving them a reddish appearance.[How to reference and link to summary or text]
Diagnosis[]
Porphyrin studies[]
Porphyria is diagnosed through spectroscopy and biochemical analysis of blood, urine, and stool.[6] In general, urine estimation of porphobilinogen (PBG) is the first step if acute porphyria is suspected. As a result of feedback, the decreased production of heme leads to increased production of precursors, PBG being one of the first substances in the porphyrin synthesis pathway.[7] In nearly all cases of acute porphyria syndromes, urinary PBG is markedly elevated except for the very rare ALA dehydratase deficiency or in patients with symptoms due to hereditary tyrosinemia type I. [How to reference and link to summary or text] In cases of mercury- or arsenic poisoning-induced porphyria, other changes in porphyrin profiles appear, most notably elevations of uroporphyrins I&III, coproporphyrins I&III and pre-coproporphyrin. [8]
Repeat testing during an attack and subsequent attacks may be necessary in order to detect a porphyria, as levels may be normal or near-normal between attacks. The urine screening test has been known to fail in the initial stages of a severe life threatening attack of acute intermittent porphyria.[How to reference and link to summary or text]
The bulk (up to 90%) of the genetic carriers of the more common, dominantly inherited acute hepatic porphyrias (acute intermittent porphyria, hereditary coproporphyria, variegate porphyria) have been noted in DNA tests to be latent for classic symptoms and may require DNA or enzyme testing. The exception to this may be latent post-puberty genetic carriers of hereditary coproporphyria.[How to reference and link to summary or text]
As most porphyrias are rare conditions, general hospital labs typically do not have the expertise, technology or staff time to perform porphyria testing. In general, testing involves sending samples of blood, stool and urine to a reference laboratory.[6] All samples to detect porphyrins must be handled properly. Samples should be taken during an acute attack, otherwise a false negative result may occur. Samples must be protected from light and either refrigerated or preserved.[6]
If all the porphyrin studies are negative, one has to consider pseudoporphyria. A careful medication review often will find the inciting cause of pseudoporphyria.
Additional tests[]
Further diagnostic tests of affected organs may be required, such as nerve conduction studies for neuropathy or an ultrasound of the liver. Basic biochemical tests may assist in identifying liver disease, hepatocellular carcinoma, and other organ problems.
Pathogenesis[]
In humans, porphyrins are the main precursors of heme, an essential constituent of hemoglobin, myoglobin, catalase, peroxidase, respiratory and P450 liver cytochromes.
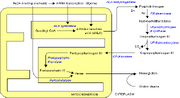
Heme synthesis—note that some reactions occur in the cytoplasm and some in the mitochondrion (yellow)
Deficiency in the enzymes of the porphyrin pathway leads to insufficient production of heme. Heme function plays a central role in cellular metabolism. This is not the main problem in the porphyrias; most heme synthesis enzymes—even dysfunctional enzymes—have enough residual activity to assist in heme biosynthesis. The principal problem in these deficiencies is the accumulation of porphyrins, the heme precursors, which are toxic to tissue in high concentrations. The chemical properties of these intermediates determine the location of accumulation, whether they induce photosensitivity, and whether the intermediate is excreted (in the urine or feces).
There are eight enzymes in the heme biosynthetic pathway, four of which—the first one and the last three—are in the mitochondria, while the other four are in the cytosol. Defects in any of these can lead to some form of porphyria.
The hepatic porphyrias are characterized by acute neurological attacks (seizures, psychosis, extreme back and abdominal pain and an acute polyneuropathy), while the erythropoietic forms present with skin problems, usually a light-sensitive blistering rash and increased hair growth.
Variegate porphyria (also porphyria variegata or mixed porphyria), which results from a partial deficiency in PROTO oxidase, manifests itself with skin lesions similar to those of porphyria cutanea tarda combined with acute neurologic attacks. All other porphyrias are either skin- or nerve-predominant.
Subtypes[]
Subtypes of porphyrias depend on what enzyme is deficient.
| Enzyme | Location of enzyme | Associated porphyria | Type of porphyria | Inheritance | Symptoms |
|---|---|---|---|---|---|
| δ-aminolevulinate (ALA) synthase | Mitochondrion | X-linked sideroblastic anemia (XLSA) | Erythropoietic | X-linked | |
| δ-aminolevulinate (ALA) dehydratase | Cytosol | Doss porphyria/ALA dehydratase deficiency | Hepatic | Autosomal recessive [9] | Abdominal pain, neuropathy[9] |
| hydroxymethylbilane (HMB) synthase (or PBG deaminase) | Cytosol | acute intermittent porphyria (AIP) | Hepatic | Autosomal dominant [9] | Periodic abdominal pain, peripheral neuropathy, psychiatric disorders, tachycardia[9] |
| uroporphyrinogen (URO) synthase | Cytosol | Congenital erythropoietic porphyria (CEP) | Erythropoeitic | Autosomal recessive [9] | Severe photosensitivity with erythema, swelling and blistering. Hemolytic anemia, splenomegaly[9] |
| uroporphyrinogen (URO) decarboxylase | Cytosol | Porphyria cutanea tarda (PCT) | Hepatic | Autosomal dominant [9] | Photosensitivity with vesicles and bullae[9] |
| coproporphyrinogen (COPRO) oxidase | Mitochondrion | Hereditary coproporphyria (HCP) | Hepatic | Autosomal dominant [9] | Photosensitivity, neurologic symptoms, colic[9] |
| protoporphyrinogen (PROTO) oxidase | Mitochondrion | Variegate porphyria (VP) | Mixed | Autosomal dominant [9] | Photosensitivity, neurologic symptoms, developmental delay |
| Ferrochelatase | Mitochondrion | Erythropoietic protoporphyria (EPP) | Erythropoietic | Autosomal dominant [9] | Photosensitivity with skin lesions. Gallstones, mild liver dysfunction[9] |
Treatment[]
Acute porphyria[]
- Carbohydrates and heme
Often, empirical treatment is required if the diagnostic suspicion of a porphyria is high since acute attacks can be fatal. A high-carbohydrate diet is typically recommended; in severe attacks, a glucose 10% infusion is commenced, which may aid in recovery.
Hematin and haem arginate are the drugs of choice in acute porphyria, in the United States and the United Kingdom, respectively. These drugs need to be given very early in an attack to be effective; effectiveness varies amongst individuals. They are not curative drugs but can shorten attacks and reduce the intensity of an attack. Side effects are rare but can be serious. These heme-like substances theoretically inhibit ALA synthase and hence the accumulation of toxic precursors. In the United Kingdom, supplies of this drug are maintained at two national centers. In the United States, one company manufactures Panhematin for infusion. The American Porphyria Foundation has information regarding the quick procurement of the drug.[10]
Any sign of low blood sodium (hyponatremia) or weakness should be treated with the addition of hematin or heme arginate or even Tin Mesoporphyrin as these are signs of impending syndrome of inappropriate antidiuretic hormone (SIADH) or peripheral nervous system involvement that may be localized or severe progressing to bulbar paresis and respiratory paralysis.[How to reference and link to summary or text]
- Precipitating factors
If drugs or hormones have caused the attack, discontinuing the offending substances is essential. Infection is one of the top causes of attacks and requires vigorous treatment.
- Symptom control
Pain is extremely severe, frequently out of proportion to physical signs and almost always requires the use of opiates to reduce it to tolerable levels. Pain should be treated early as medically possible due to its severity. Nausea can be severe; it may respond to phenothiazine drugs but is sometimes intractable. Hot water baths/showers may lessen nausea temporarily, though caution should be used to avoid burns or falls.
- Early identification
Patients with a history of acute porphyria and even genetic carriers are recommended to wear an alert bracelet or other identification at all times in case they develop severe symptoms or in case of accidents where there is a potential for drug exposure: a result of which may be they cannot explain to healthcare professionals about their condition and the fact that some drugs are absolutely contraindicated.
- Neurologic and psychiatric issues
Patients who experience frequent attacks can develop chronic neuropathic pain in extremities as well as chronic pain in the gut. Gut dysmotility, ileus, intussusception, hypoganglionosis, encopresis in children and intestinal pseudo-obstruction have been associated with porphyrias. This is thought to be due to axonal nerve deterioration in affected areas of the nervous system and vagal nerve dysfunction.
In these cases treatment with long-acting opioids may be indicated. Some cases of chronic pain can be difficult to manage and may require treatment using multiple modalities. Opioid dependence may develop.
Depression often accompanies the disease and is best dealt with by treating the offending symptoms and if needed the judicious use of anti-depressants. Some psychotropic drugs are porphyrinogenic, limiting the pharmacotherapeutic scope.
- Seizures
Seizures often accompany this disease. Most seizure medications exacerbate this condition. Treatment can be problematic: barbiturates especially must be avoided. Some benzodiazepines are safe, and, when used in conjunction with newer anti-seizure medications such as gabapentin offer a possible regime for seizure control.
Magnesium sulfate and bromides have also been used in porphyria seizures, however, development of status epilepticus in porphyria may not respond to magnesium alone. The addition of hematin or heme arginate has been used during status epilepticus.[How to reference and link to summary or text]
- Underlying liver disease
Some liver diseases may cause porphyria even in the absence of genetic predisposition. These include hemochromatosis and hepatitis C. Treatment of iron overload may be required.
- Hormone treatment
Hormonal fluctuations that contribute to cyclical attacks in women have been treated with oral contraceptives and luteinizing hormones to shut down menstrual cycles. However, oral contraceptives have also triggered photosensitivity and withdrawal of oral contraceptives has triggered attacks. Androgens and fertility hormones have also triggered attacks.
Erythropoietic porphyrias[]
These are associated with accumulation of porphyrins in erythrocytes and are rare. The rarest is Congenital erythropoetic porphyria (C.E.P) otherwise known as Gunther's disease. Its rarity is partially due to its autosomal recessive mode of inheritance. The signs may present from birth and include severe photosensitivity, brown teeth that fluoresce in ultraviolet light due to deposition of type one porphyrins and later hypertrichosis. Haemolytic anaemia usually develops. Pharmaceutical-grade beta carotene may be used in its treatment.[11] A bone marrow transplant has also been successful in curing CEP in a few cases, although long term results are not yet available.[12]
The pain, burning, swelling and itching that occur in erythropoietic porphyrias generally require avoidance of bright sunlight. Most kinds of sunscreen are not effective, but SPF-rated long-sleeve shirts, hats, bandanas and gloves can help. Chloroquine may be used to increase porphyrin secretion in some EPs.[6] Blood transfusion is occasionally used to suppress innate heme production.
Culture and history[]
Porphyrias have been detected in all races, multiple ethnic groups on every continent including Caucasians, Asians, Africans, Peruvian/Mexican Hispanics, Native Americans, Laplanders and Australian aborigines. There are high incidence reports of AIP in areas of India and Scandinavia and over 200 genetic variants of AIP, some of which are specific to families, although some strains have proven to be repeated mutations.
The Scandinavian source of porphyria has been traced to the Sámi ethnic group. Their language, as well as the languages of Finland, Estonia, Hungary, Transylvania, and Bulgaria have ties to languages in small groups of people living in Russia on both sides of the Urals and are branches of Uralic languages and Altaic languages (the Finno-Ugric Languages).
The links between porphyrias and mental disorders have been noted for decades. In the early 1950's patients with porphyrias (occasionally referred to as "Porphyric Hemophilia"[13]) and severe symptoms of depression or catatonia were treated with electroshock.
Historical patients[]
The insanity exhibited by King George III evidenced in the regency crisis of 1788 has inspired several attempts at retrospective diagnosis. The first, written in 1855, thirty-five years after his death, concluded he suffered from acute mania. M. Guttmacher, in 1941, suggested manic-depressive psychosis as a more likely diagnosis, The first suggestion that a physical illness was the cause of King George's mental derangements came in 1966, in a paper "The Insanity of King George III: A Classic Case of Porphyria"[14], with a follow-up in 1968, "Porphyria in the Royal Houses of Stuart, Hanover and Prussia"[15]. The papers, by a mother/son psychiatrist team, were written as though the case for porphyria had been proven, but the response demonstrated that many, including those more intimately familiar with actual manifestations of porphyria, were unconvinced. The theory is treated in Purple Secret[16], which documents the ultimately unsuccessful search for genetic evidence of porphyria in the remains of royals suspected to suffer from it.[17] In 2005 it was suggested that arsenic (which is known to be porphyrogenic) given to George III with antimony may have caused his porphyria.[18] Despite the lack of direct evidence, the notion that George III (and other members of the royal family) suffered from porphyria has achieved such popularity that many forget that it is merely a hypothesis.
Other commentators have suggested that Vincent van Gogh may have suffered from acute intermittent porphyria.[19]
It has also been imagined that King Nebuchadnezzar of Babylon suffered from some form of porphyria (cf. Daniel 4).[20] The symptoms of the various porphyrias are so wide-ranging that nearly any constellation of symptoms can be attributed to one or more of them.[How to reference and link to summary or text]
See also[]
References[]
- ↑ 1.0 1.1 Nick Lane. Born to the purple: the story of porphyria. Scientific American. URL accessed on 2008-08-05.
- ↑ Hoppe-Seyler F (1871). Das Hämatin. Tubinger Med-Chem Untersuch 4: 523–33.
- ↑ Stokvis BJ. Over twee zeldzame kleurstoffen in urine van zieken. Nederl Tijdschr Geneeskd 2: 409–417. Reprinted in Stokvis BJ (December 1989). Over twee zeldzame kleurstoffen in urine van zieken. Ned Tijdschr Geneeskd 133 (51): 2562–70.
- ↑ Albers JW, Fink JK (2004). Porphyric neuropathy. Muscle Nerve 30 (4): 410–22.
- ↑ Roelandts R (2000). The diagnosis of photosensitivity. Arch Dermatol 136 (9): 1152–7.
- ↑ 6.0 6.1 6.2 6.3 Thadani H, Deacon A, Peters T (2000). Diagnosis and management of porphyria. BMJ 320 (7250): 1647–51.
- ↑ Anderson KE, Bloomer JR, Bonkovsky HL, et al (2005). Recommendations for the diagnosis and treatment of the acute porphyrias. Ann. Intern. Med. 142 (6): 439–50.
- ↑ Woods, J.S. (1995), "Porphyrin metabolism as indicator of metal exposure and toxicity", in Goyer, R.A. & Cherian, M.G., Toxicology of metals, biochemical aspects, 115, Berlin: Springer, pp. 19–52, Chapter 2
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 Table 18-1 in: Marks, Dawn B.; Swanson, Todd; Sandra I Kim; Marc Glucksman (2007). Biochemistry and molecular biology, Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
- ↑ American Porphyria Foundation - Panhematin Quick Delivery Fulltext
- ↑ Martin A Crook.2006. Clinical chemistry and Metabolic Medicine. seventh edition. Hodder Arnold. ISBN 0-340-90616-2
- ↑ Faraci M, Morreale G, Boeri E, et al (2008). Unrelated HSCT in an adolescent affected by congenital erythropoietic porphyria. Pediatr Transplant 12 (1): 117–20.
- ↑ Denver, Joness. "An Encyclopaedia of Obscure Medicine". Published by University Books, Inc., 1959.
- ↑ Ida Macalpine & Richard Hunger, "The Insanity of King George III: A Classic Case of Porphyria", British Medical Journal, 1966, pp. 65-71.
- ↑ Ida Macalpine, Richard Hunger, & Claude Rimington, "Porphyria in the Royal Houses of Stuart, Hanover and Prussia: A Followup Study of George III's Illness", British Medical Journal, 1968, pp. 7-18.
- ↑ Röhl, John C.G., Warren Martin,& David Hunt, Purple Secret, Bantam Press, London, 1998 ISBN 0-593-04148-8
- ↑ The authors demonstrated a single point mutation in the PPOX gene, but not one which has been associated with disease.
- ↑ Cox TM, Jack N, Lofthouse S, Watling J, Haines J, Warren MJ. King George III and porphyria: an elemental hypothesis and investigation. Lancet 2005;366(9482):332-5. PMID 16039338.
- ↑ Loftus LS, Arnold WN. Vincent van Gogh's illness: acute intermittent porphyria? BMJ 1991;303:1589-91. PMID 1773180.
- ↑ Beveridge A. The madness of politics. J R Soc Med 2003;96:602-4. PMID 14645615.
- Kauppinen R. Porphyrias. Lancet 2005;365:241-52. PMID 15652607.
Further reading[]
Books[]
- Roth, N. (1987). Psychiatric syndromes of porphyria. New York, NY: W W Norton & Co.
Papers[]
- Auchincloss, S., & Pridmore, S. (2001). Vomiting, burns and irrational behaviour: Lancet Vol 358(9296) Dec 2001, 1870.
- Beber, E., & Cooper, S.-A. (1998). Depression and porphyria: Safe use of fluoxetine with a woman with learning disabilities: International Journal of Psychiatry in Clinical Practice Vol 2(4) Dec 1998, 303-305.
- Boon, F. F., & Ellis, C. (1989). Acute intermittent porphyria in a children's psychiatric hospital: Journal of the American Academy of Child & Adolescent Psychiatry Vol 28(4) Jul 1989, 606-609.
- Bronckart, C., & Troisfontaines, B. (1984). Acute porphyrias and psychiatry: Updating of our knowledge: Acta Psychiatrica Belgica Vol 84(4) Jul-Aug 1984, 336-352.
- Brownstein, S. (1997). George III: A revised view of the royal malady: Journal of the History of the Neurosciences Vol 6(1) Apr 1997, 38-49.
- Burgoyne, K., Swartz, R., & Ananth, J. (1995). Porphyria: Reexamination of psychiatric implications: Psychotherapy and Psychosomatics Vol 64(3-4) 1995, 121-130.
- Chinnery, P. F., Cartlidge, N., Burn, D. J., Cleland, P. G., & McKeith, I. (1997). Management of parkinsonism and psychotic depression in a case of acute intermittent porphyria: Journal of Neurology, Neurosurgery & Psychiatry Vol 62(5) May 1997, 542.
- Crimlisk, H. L. (1997). The little imitator--porphyria: A neuropsychiatric disorder: Journal of Neurology, Neurosurgery & Psychiatry Vol 62(4) Apr 1997, 319-328.
- Croarkin, P. (2002). From King George to neuroglobin: The psychiatric aspects of acute intermittent porphyria: Journal of Psychiatric Practice Vol 8(6) Nov 2002, 398-405.
- Diot, E., Corcia, P., Zannad, N., Chauvet, M. A., Borie, M. J., & Maillot, F. (2007). Favorable outcome of acute porphyric neuropathy after treatment with heme arginate: Revue Neurologique Vol 163(11) Nov 2007, 1100-1102.
- Dover, S. B., Graham, A., Moore, M. R., McColl, K. E. L., & et al. (1994). Lofepramine: A safe anti-depressant in acute hepatic porphyria? : Journal of Psychopharmacology Vol 8(2) 1994, 104-108.
- Ellencweig, N., Schoenfeld, N., & Zemishlany, Z. (2006). Acute Intermittent Porphyria: Psychosis as the Only Clinical Manifestation: Israel Journal of Psychiatry and Related Sciences Vol 43(1) 2006, 52-56.
- Engelhardt, K., Trinka, E., Franz, G., Unterberger, I., Spiegel, M., Beer, R., et al. (2004). Refractory status epilepticus due to acute hepatic porphyria in a pregnant woman: Induced abortion as the sole therapeutic option? : European Journal of Neurology Vol 11(10) Oct 2004, 693-697.
- Fornazzari, L., Remington, G., & Jeffries, J. (1991). Akathisia, porphyria and low iron: The Canadian Journal of Psychiatry / La Revue canadienne de psychiatrie Vol 36(7) Sep 1991, 548.
- Gaida-Hommernick, B., Rieck, K., & Runge, U. (2001). Oxcarbazepine in focal epilepsy and hepatic porphyria: A case report: Epilepsia Vol 42(6) Jun 2001, 793-795.
- Gibbons, D., Cullen, A., & Garland, M. (2003). Porphyria and dementia: A case report: Irish Journal of Psychological Medicine Vol 20(3) Sep 2003, 96-99.
- Gibney, G. N., Jones, I. H., & Meek, J. H. (1972). Schizophrenia in association with Erythropoietic Protoporphyria: Report of a case: British Journal of Psychiatry Vol 121(560) Jul 1972, 79-81.
- Ginsberg, D. L. (2007). Acute porphyria triggered by duloxetine: Primary Psychiatry Vol 14(4) Apr 2007, 31.
- Golechha, G. R. (1989). Acute hepatic porphyria and psychoses (experience of twelve years): Indian Journal of Psychiatry Vol 31(4) Oct 1989, 319-328.
- Gonzalez-Arriaza, H. L., & Bostwick, J. M. (2003). Acute porphyrias: A case report and review: American Journal of Psychiatry Vol 160(3) Mar 2003, 450-459.
- Grabowski, J., & Yeragani, V. K. (1987). Porphyria and psychosis: A case report: The Canadian Journal of Psychiatry / La Revue canadienne de psychiatrie Vol 32(5) Jun 1987, 393-394.
- Hamner, M. B. (1992). Obsessive-compulsive symptoms associated with acute intermittent porphyria: Psychosomatics: Journal of Consultation Liaison Psychiatry Vol 33(3) Sum 1992, 329-331.
- Horgan, P., & Jones, H. (2003). Olanzapine use in acute porphyria: International Journal of Psychiatry in Clinical Practice Vol 7(1) Mar 2003, 67-69.
- Irvine, D. G. (1983). Identification, chemistry and clinical correlates of a pyrrolic metabolite in neuropsychiatric disorders: Dissertation Abstracts International.
- Kaplan, P. W., & Lewis, D. V. (1986). Juvenile acute intermittent porphyria with hypercholesterolemia and epilepsy: A case report and review of the literature: Journal of Child Neurology Vol 1(1) Jan 1986, 38-45.
- King, P. H., & Bragdon, A. C. (1991). MRI reveals multiple reversible cerebral lesions in an attack of acute intermittent porphyria: Neurology Vol 41(8) Aug 1991, 1300-1302.
- Lemmens, L., & Hougardy, G. (1990). Porphyria and psychiatric symptomatology: A case report: Acta Psychiatrica Belgica Vol 90(2) Mar-Apr 1990, 100-111.
- Loper, T., & Touchet, B. (2007). An Acute Attack of Porphyria in a Patient Taking Duloxetine: Psychosomatics: Journal of Consultation Liaison Psychiatry Vol 48(2) Mar-Apr 2007, 179-180.
- Mandoki, M. W., & Sumner, G. S. (1994). Psychiatric manifestations of hereditary coproporphyria in a child: Journal of Nervous and Mental Disease Vol 182(2) Feb 1994, 117-118.
- Mercan, S., Karamustafalioglu, O., Tanriverdi, N., & Oba, S. (2003). Safety of fluoxetine treatment in a case of acute intermittent porphyria: International Journal of Psychiatry in Clinical Practice Vol 7(4) Dec 2003, 281-283.
- Miller, P. R. (1995). Diagnosis and serendipity: American Journal of Psychiatry Vol 152(10) Oct 1995, 1530.
- No authorship, i. (1979). MMPI profile of porphyria: Clinical Neuropsychology Vol 1(2) 1979, 32.
- Parry, J. Y., Allanic, A., & Ballon, C. (1974). Intermittent mental disturbances and porphyria: Annales Medico-Psychologiques Vol 1(1) Jan 1974, 137-140.
- Patience, D. A., Blackwood, D. H. R., McColl, K. E. L., & Moore, M. R. (1994). Acute intermittent porphyria and mental illness: A family study: Acta Psychiatrica Scandinavica Vol 89(4) Apr 1994, 262-267.
- Procter, E. A., & McKenzie, I. P. (1994). Opiate dependence in acute intermittent porphyria: Irish Journal of Psychological Medicine Vol 11(3) Sep 1994, 133-134.
- Regan, L., Gonsalves, L., & Tesar, G. (1999). Acute intermittent prophyria: Psychosomatics: Journal of Consultation Liaison Psychiatry Vol 40(6) Nov-Dec 1999, 521-523.
- Rijn-van den Meijdenberg, J. C. C., Fekkes, D., Wilson, J. H. P., Pepplinkhuizen, L., & et al. (1994). Acute intermittent porphyria and disturbances in amino-acid metabolism in a psychiatric in-patient population: European Psychiatry Vol 9(5) 1994, 249-253.
- Santosh, P. J., & Malhotra, S. (1994). Varied psychiatric manifestations of acute intermitttent porphyria: Biological Psychiatry Vol 36(11) Dec 1994, 744-747.
- Schouten, M. J., & Bruinvels, J. (1986). Endogenously formed norharman (!b-carboline) in platelet rich plasma obtained from porphyric rats: Pharmacology, Biochemistry and Behavior Vol 24(5) May 1986, 1219-1223.
- Schouten, M. J., Bruinvels, J., Pepplinkhuizen, L., & Wilson, J. P. (1983). Serine and glycine-induced catalepsy in porphyric rats: An animal model for psychosis? : Pharmacology, Biochemistry and Behavior Vol 19(2) Aug 1983, 245-250.
- Strauss, J., & DiMartini, A. (1999). Use of olanzapine in hereditary coproporphyria: Psychosomatics: Journal of Consultation Liaison Psychiatry Vol 40(5) Sep-Oct 1999, 444-445.
- Thies, M., & Schaub, H. (1977). Auditory hallucinosis in acute intermittent porphyria: Nervenarzt Vol 48(2) Feb 1977, 89-90.
- Thunell, S., Floderus, Y., Henrichson, A., Moore, M. R., & et al. (1992). Alcoholic beverages in acute porphyria: Journal of Studies on Alcohol Vol 53(3) May 1992, 272-276.
- Tishler, P. V., & et al. (1985). High prevalence of intermittent acute porphyria in a psychiatric patient population: American Journal of Psychiatry Vol 142(12) Dec 1985, 1430-1436.
- Urban, S. (1985). Psychic disturbances in acute intermittent porphyria: Psychiatria Polska Vol 19(3) May-Jun 1985, 233-238.
- Vaz, F. J., & Salcedo, M. S. (1991). Fluoxetine treatment of depressive symptoms in acute intermittent porphyria: Journal of Clinical Psychiatry Vol 52(3) Mar 1991, 138.
- Vgontzas, A. N., Kales, J. D., Ballard, J. O., Vela-Bueno, A., & et al. (1993). Porphyria and panic disorder with agoraphobia: Psychosomatics: Journal of Consultation Liaison Psychiatry Vol 34(5) Sep-Oct 1993, 440-443.
- Vos, A. M., Holslag, I. M., Snelleman, W., & Hoek, H. W. (2003). Acute porphyria: an intriguing imitator: Tijdschrift voor Psychiatrie Vol 45(5) 2003, 277-286.
- Wessels, T., Blaes, F., Rottger, C., Hugens, M., Huge, S., & Jauss, M. (2005). Cortical amaurosis and status epilepticus with acute porphyria: Nervenarzt Vol 76(8) Aug 2005, 992-998.
- Winkler, A. S., Peters, T. J., & Elwes, R. D. C. (2005). Neuropsychiatric porphyria in patients with refractory epilepsy: Report of three cases: Journal of Neurology, Neurosurgery & Psychiatry Vol 76(3) Mar 2005, 380-383.
- Zaatreh, M. M. (2005). Levetiracetam in porphyric status epilepticus: A case report: Clinical Neuropharmacology Vol 28(5) Sep-Oct 2005, 243-244.
External links[]
- American Porphyria Foundation
- European Porphyria Initiative
- Porphynet - site on porphyrins and the porphyrias
- The Drug Database for Acute Porphyria - comprehensive database on drug porphyrinogenicity
amino-acids Phenylketonuria - Alkaptonuria - Ochronosis - Tyrosinemia - Maple syrup urine disease - Propionic acidemia - Methylmalonic acidemia - Isovaleric acidemia - Primary carnitine deficiency - Cystinuria - Cystinosis - Hartnup disease - Homocystinuria - Citrullinemia - Hyperammonemia - Glutaric acidemia type 1
carbohydrates Lactose intolerance - Glycogen storage disease (type I, type II, type III, type IV, type V), Fructose intolerance, Galactosemia
Lipid storage disorders Gangliosidosis - GM2 gangliosidoses (Sandhoff disease, Tay-Sachs disease) - GM1 gangliosidoses - Mucolipidosis type IV - Gaucher's disease - Niemann-Pick disease - Farber disease - Fabry's disease - Metachromatic leukodystrophy - Krabbe disease - Neuronal ceroid lipofuscinosis - Batten disease - Cerebrotendineous xanthomatosis - Wolman disease - Cholesteryl ester storage disease
List of fatty acid metabolism disorders - Hyperlipidemia - Hypercholesterolemia - Familial hypercholesterolemia - Xanthoma - Combined hyperlipidemia - Lecithin cholesterol acyltransferase deficiency - Tangier disease - Abetalipoproteinemia
mineral metabolism Disorders of calcium metabolism - Hypophosphatemia - Hypophosphatasia - Wilson's disease - Menkes disease - Hypermagnesemia - Hypomagnesemia - Hypercalcaemia - Hypocalcaemia
fluid, electrolyte and acid-base balance Electrolyte disturbance - Hypernatremia - Hyponatremia - Respiratory acidosis - Metabolic acidosis - Lactic acidosis - Hypervolemia - Hypokalemia - Hyperkalemia - Mixed disorder of acid-base balance - Hyperchloremia - Hypochloremia - Dehydration
porphyrin and bilirubin Acatalasia - Gilbert's syndrome - Crigler-Najjar syndrome - Dubin-Johnson syndrome - Rotor syndrome - Porphyria (Acute intermittent porphyria, Gunther's disease, Porphyria cutanea tarda, Erythropoietic protoporphyria, Hepatoerythropoietic porphyria, Hereditary coproporphyria, Variegate porphyria)
glycosaminoglycan Mucopolysaccharidosis - Hurler syndrome - Hunter syndrome - Sanfilippo syndrome - Morquio syndrome
glycoprotein I-cell disease - Pseudo-Hurler polydystrophy - Aspartylglucosaminuria - Fucosidosis - Alpha-mannosidosis - Sialidosis
other Alpha 1-antitrypsin deficiency - Cystic fibrosis - Familial Mediterranean fever - Lesch-Nyhan syndrome
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |