Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
| DOI | |
|---|---|
| Chemical name | 1-(2,5-dimethoxy-4-iodophenyl)- propan-2-amine |
| Chemical formula | C11H16INO2 |
| Molecular mass | 321.15 g/mol |
| Melting point | 201 °C (hydrochloride) |
| CAS numbers | 64584-34-5, 82830-53-3, 82864-06-0, 99665-04-0 |
| SMILES | N[C@H](C)CC1=C(OC)C=C(I)C(OC)=C1 (R-isomer) |
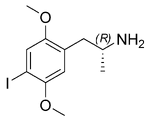 
| |
DOI or 2,5-dimethoxy-4-iodoamphetamine is a psychedelic hallucinogenic drug and a substituted amphetamine of the phenethylamine family. Despite being a substituted amphetamine it is not a stimulant. DOI has a stereocenter and R-(-)-DOI is the more active stereoisomer. [125I]-R-(-)-DOI is used as a radioligand. DOI is active at a dosage of 1.5 - 3.0 mg and has an extremely long duration of 16 - 30 hours. DOI is used recreationally, and is often confused with LSD by inexperienced users. However, the trip is somewhat more energetic than an LSD trip, and often the visuals are less intense. The after effects include a difficulty getting to sleep and communicating. Depending on the amount taken, these symptoms may persist for days.
Pharmacology[]
The hallucinogenic effect of DOI is mediated by its partial agonistic activity at the 5-HT2A serotonin receptor, but DOI also has a high binding affinity for the 5-HT2B and the 5-HT2C serotonin receptors. DOI is often used as a tool compound in research when studying the 5-HT2 receptor family. The solubility of DOI-hydrochloride in H2O is 10 mg/ml, and in ethanol 2 mg/ml (source: www.sigmaaldrich.com MSDS)
History[]
DOI was first synthesized by Alexander Shulgin. The radioactive iodine-125 form of DOI was first developed in the lab of David E. Nichols. British Police recently reported that 3 young men had taken ill, reportedly, after taking DOI at a rave in Biggleswade, near Milton Keynes, and warned others who had taken it to seek medical attention. This would appear to the first indication that DOI has found more widespread use as a recreational drug in the UK. [1]
See also[]
References[]
- ↑ BBC, "New drug alert as three taken ill", BBC NEWS, 29 January 2007.
External[]
Categorization[]
Psychedelic phenethylamines
| |
|---|---|
Aleph • 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-O • 2C-O-4 • 2C-P • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 3C-E • 3C-P • Br-DFLY • DESOXY • DMMDA-2 • DOB • DOC • DOET • DOI • DOM • DON • Escaline • Ganesha • HOT-2 • HOT-7 • HOT-17 • Isoproscaline • Lophophine • MDA • MMDA • MMDA-2 • MMDA-3a • MMDMA • Macromerine • Mescaline • Proscaline • TMA |
.
- pl:DOI
- sv:DOI
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |