Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
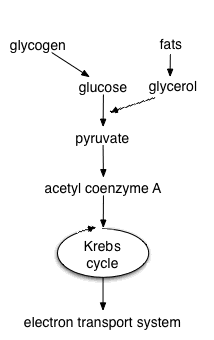
Cellular respiration is the process in which the chemical bonds of energy-rich molecules such as glucose are converted into energy usable for life processes. Oxidation of organic material—in a bonfire, for example—is an exothermic reaction that releases a large amount of energy rather quickly. The equation for the oxidation of glucose is:
- C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy released (2830 kJ)
In a fire there is a massive uncontrolled release of energy as light and heat. Cellular respiration is the same process but it occurs in gradual steps that result in the conversion of the energy stored in glucose to usable chemical energy in the form of ATP.
Aerobic respiration[]
Aerobic respiration requires oxygen in order to generate energy. It is the preferred method of pyruvate breakdown from glycolysis and requires that pyruvate enter the mitochondrion to be fully oxidized by the Krebs cycle. The product of this process is energy in the form of ATP (Adenosine Triphosphate), by substrate-level phosphorylation, NADH and FADH2. The reducing potential of NADH and FADH2 is converted to more ATP via an electron transport chain with oxygen as the "terminal electron acceptor". Most of the ATP produced by cellular respiration is by oxidative phosphorylation, ATP molecules are made due to the chemiosmotic potential driving ATP synthase. Respiration is the process by which cells obtain energy when oxygen is present in the cell.
Theoretically, 36 ATP molecules can be made per glucose during cellular respiration, however, such conditions are generally not realized due to such losses as the cost of moving pyruvate into mitochondria. Aerobic metabolism is rather more efficient than anaerobic metabolism. They share the initial pathway of glycolysis but aerobic metabolism continues with the Krebs cycle and oxidative phosphorylation. The post glycolytic reactions take place in the mitochondria in eukaryotic cells, and at the cell membrane in prokaryotic cells.
Glycolysis[]
- Main article: Glycolysis
Glycolysis is a metabolic pathway that is found in all living organisms and does not require oxygen. The process converts one molecule of glucose into two molecules of pyruvate, and makes energy in the form of two net molecules of ATP. Four molecules of ATP per glucose are actually produced but two are consumed for the preparatory phase. The initial phosphorylation of glucose is required to destablise the molecule for cleavage into two triose sugars. During the pay-off phase of glycolysis four phosphate groups are transfered to ADP by substrate-level phosphorylation to make four ATP and two NADH are produced when the triose sugars are oxidised. Glycolysis takes place in the cytoplasm of the cell. The overall reaction can be expressed this way:
- Glucose + 2 ATP + 2 NAD+ + 2 Pi + 4 ADP → 2 pyruvate + 2 ADP + 2 NADH + 4 ATP + 2 H2O + 4 H+
Oxidative decarboxylation[]
- Main article: Oxidative decarboxylation
Produces acetyl-CoA from pyruvate. This oxidation reaction also releases carbon dioxide as a product.
Krebs cycle/Citric Acid cycle[]
- Main article: Citric acid cycle
When oxygen is present, acetyl-CoA enters the citric acid cycle, and gets oxidised to CO2 while at the same time reducing NAD to NADH. NADH can be used by the electron transport chain to create further ATP as part of oxidative phosphorylation. To fully oxidise the equivalent of one glucose molecule two acetyl-CoA must be metabolised by the Krebs cycle. Two waste products, H2O and CO2 are created during this cycle, .
Oxidative phosphorylation[]
Main articles: Oxidative phosphorylation , Electron transport chain , Electrochemical gradient, and ATP synthase
In eukaryotes, oxidative phosphorylation occurs in the mitochondria. It comprises of the electron transport chain that establishes a proton gradient (chemiosmotic potential) across the inner membrane by oxidising the NADH produced from the Krebs cycle. ATP is synthesised by the ATP synthase enzyme when the chemiosmotic gradient is used to drive the phosphorylation of ADP.
Theoretical yields[]
The yields in the table below are for one glucose and molecule being fully oxidised to carbon dioxide. It is assumed that all the reduced coenzymes are oxidised by the electron transport chain and used for oxidative phosphorylation.
| Step | coenzyme yield | ATP yield | Source of ATP |
|---|---|---|---|
| Glycolysis preparatory phase | -2 | Phosphorylation of glucose and fructose 6-phosphate uses two ATP from the cytoplasm. | |
| Glycolysis pay-off phase | 4 | Substrate-level phosphorylation | |
| 2 NADH | 4 | Oxidative phosphorylation. Only 2 ATP per NADH since the coenzyme must feed into the electron transport chain from the cytoplasm rather than the mitochondrial matrix. | |
| Oxidative carboxylation | 2 NADH | 6 | Oxidative phosphorylation |
| Krebs cycle | 2 | Substrate-level phosphorylation | |
| 6 NADH | 18 | Oxidative phosphorylation | |
| 2 FADH2 | 4 | Oxidative phosphorylation | |
| Total yield | 36 | From the complete oxidation of one glucose molecule to carbon dioxide and oxidation of all the reduced coenzymes. | |
Although there is a theoretical yield 36 ATP molecules per glucose during cellular respiration, such conditions are generally not realized due to losses such as the cost of moving pyruvate (from glycolysis), phosphate and ADP (substrates for ATP syhthesis) into the mitochondria. All are actively transported using carriers that utilise the stored energy in the proton electrochemical gradient.
- The pyruvate carrier is a symporter and the driving force for moving pyruvate into the mitochondria is the movement of protons from the inner membrane space to the matrix.
- The phosphate carrier is an antiporter and the driving force for moving phosphate ions into the mitochondria is the movement of hydroxyls ions from the matrix to the inner membrane space.
- The adenine nucleotide carrier is a symporter and exchanges ADP and ATP across the inner membrane. The driving force is due to the ATP (-4) having a more negative charge than the ADP (-3) and thus it dissipates some of the electrical component of the proton electrochemical gradient.
The outcome of these transport processes using the proton electrochemical gradient is that more than 3 H+ are needed to make 1 ATP. Obviously this reduces the theoretical efficiency of the whole process. Other factors may also dissipate the proton gradient creating an apparently leaky mitochondria. An uncoupling protein known as thermogenin is expressed in some cell types and is a channel that can transport protons. When this protein is active in the inner membrane it short circuits the coupling between the electron transport chain and ATP synthesis. The potential energy from the proton gradient is not used to make ATP but generates heat. This is particularly important in a babies brown fat, for thermogenesis, and hibernating animals.
Anaerobic respiration[]
In the absence of oxygen, pyruvate is not metabolized by cellular respiration but undergoes fermentation.
See also[]
External links[]
- Cellular respiration and Cell biology
- A detailed diagram of glycolysis
- Chart of Important Metabolic Products
- A detailed description of respiration vs. fermentation
- Interactive Molecular models of electron-transfer complexes
da:Aerob respiration de:Zellatmung fr:Respiration aérobie es:Respiración celular fr:Respiration cellulaire he:נשימה תאית sl:Celično dihanje fi:Soluhengitys zh:呼吸作用
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |