Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
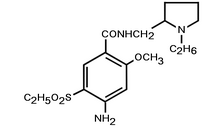
| 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]- 5-ethylsulfonyl-2-methoxy-benzamide IUPAC name | |
| CAS number 53583-79-2 |
ATC code |
| PubChem 2159 |
DrugBank none |
| Chemical formula | {{{chemical_formula}}} |
| Molecular weight | 369.48 g/mol |
| Bioavailability | 48%[1] |
| Metabolism | ? |
| Elimination half-life | 12 h[1] |
| Excretion | Renal[1] |
| Pregnancy category | {{{pregnancy_category}}} |
| Legal status | Rx-only |
| Routes of administration | Oral, intramuscular[2] |
Amisulpride (brand-name Solian®) is an antipsychotic drug sold by Sanofi-Aventis.
Amisulpride is a selective dopamine antagonist. It has a high affinity for D2 (Ki 2.8 nM) and D3 (Ki 3.2 nM) dopaminergic receptors. Its dosage ranges from 200 to 1200 mg/day. Lower doses (less than 50 mg) preferentially block d2 autoreceptors that control the synthesis and release of dopamine. This results in an increase in dopaminergic transmission. This dopamine increase is hypothesized to cause a reduction in both depressive and negative symptoms. Higher doses of the drug block the postsynaptic dopamine receptors resulting in an improvement in psychoses.
Amisulpride is not approved by the Food and Drug Administration for use in the United States. Amisulpride (in 50mg doses) is marketed as a treatment for dysthymia in Italy (as Deniban) In one study, anxiety measured by HAM-A total mean score decreased significantly more with amisulpride 50mg/day (63%) than with fluoxetine 20mg/day (54%; P = 0.021).[3]
Side effects[]
Prolactin induction, nausea, weight gain, although much less than similar drugs in its class, and less commonly QT interval prolongation (which can lead to serious heart arrhythmias). Overdoses of amisulpride have been linked with torsades de pointes.[4]
See also[]
References[]
- ↑ 1.0 1.1 1.2 Rosenzweig P, Canal M, Patat A, Bergougnan L, Zieleniuk I, Bianchetti G. (2002). A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers.. Human Psychopharmacology 17 (1): 1-13.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedcas_biam - ↑ Smeraldi E (1998). Amisulpride versus fluoxetine in patients with dysthymia or major depression in partial remission: a double-blind, comparative study. J Affect Disord 48 (1): 47-56.
- ↑ Isbister G, Murray L, John S, Hackett L, Haider T, O'Mullane P, Gosselin S, Daly F (2006). Amisulpride deliberate self-poisoning causing severe cardiac toxicity including QT prolongation and torsades de pointes. Med J Aust 184 (7): 354-6. PMID 16584372. Free full text
Psycholeptics: antipsychotics (N05A)
| |
|---|---|
| Phenothiazine typical antipsychotics | Chlorpromazine • Fluphenazine • Mesoridazine • Perphenazine • Prochlorperazine • Promazine • Thioridazine/Sulforidazine • Trifluoperazine |
| Other typical antipsychotics | Indoles (Molindone) • Butyrophenones (Azaperone, Benperidol, Droperidol, Haloperidol) • Thioxanthenes (Flupentixol, Chlorprothixene, Thiothixene, Zuclopenthixol) • diphenylbutylpiperidines (Fluspirilene, Penfluridol, Pimozide) • other (Loxapine) |
| Atypical antipsychotics | Butyrophenones (Melperone) • Indoles (Sertindole, Ziprasidone) • Benzamides (Sulpiride, Remoxipride, Amisulpride) • diazepines/oxazepines/thiazepines (Clozapine, Olanzapine, Quetiapine) • other (Aripiprazole, Risperidone, Paliperidone, Zotepine) |
- de:Amisulprid
- fr:Amisulpride
- ru:Амисульприд
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |