No edit summary |
(update wp) |
||
| Line 1: | Line 1: | ||
{{BioPsy}} |
{{BioPsy}} |
||
| + | {{For|the unfolding of an organism or the theory that plants and animals develop in this way| Epigenesis (biology)}} |
||
| − | '''Epigenetics''' is the study of [[heritability|heritable]] changes in [[gene]] function that occur without a change in the sequence of [[Cell nucleus|nuclear]] [[DNA]]. It is also the study of the processes involved in the unfolding development of an organism. In both cases, the object of study includes how gene regulatory information that is not expressed in DNA sequences is transmitted from one generation (of cells or organisms) to the next - that is (harking back to the Greek prefix), 'in addition to' the genetic information encoded in the DNA. |
||
| + | '''Epigenetics''' is a term in biology used to refer to features such as [[chromatin]] and [[DNA]] modifications that are stable over rounds of [[cell (biology)|cell]] [[mitosis|division]] but do not involve changes in the underlying [[DNA]] sequence of the organism.<ref>{{cite journal| title=Perceptions of epigenetics| author=Adrian Bird| journal=Nature| volume=447| pages=396-398| year=2007}} PMID 17522671</ref> These epigenetic changes play a role in the process of [[morphogenesis|cellular differentiation]], allowing cells to stably maintain different characteristics despite containing the same genomic material. Epigenetic features are inherited when cells divide despite a lack of change in the DNA sequence itself and, although most of these features are considered dynamic over the course of development in multicellular organisms, some epigenetic features show transgenerational inheritance and are inherited from one generation to the next.<ref>{{cite journal| title=Paramutation: From Maize to Mice| author=V.L. Chandler| journal=Cell| volume=128| pages=641-645| year=2007}}</ref> |
||
| − | ==Progress in epigenetics== |
||
| − | In recent years, there has been rapid progress in understanding epigenetic mechanisms, which include differences in [[DNA methylation]], as well as differences in [[chromatin]] structure involving [[Histone#Histone_modfications_in_chromatin_regulation|histone modifications]]. Another possibility involves the genomes of cytoplasmic elements ([[chloroplast]]s and [[mitochondrion|mitochondria]]). Other mechanisms have also been proposed. See below for more detail. |
||
| + | Specific epigenetic processes include [[paramutation]], [[bookmarking]], [[Imprinting (genetics)|imprinting]], [[gene silencing]], [[X-inactivation|X chromosome inactivation]], [[position effect]], [[reprogramming]], [[transvection (genetics)|transvection]], [[maternal effect]]s, the progress of [[carcinogenesis]], many effects of [[teratogen]]s, regulation of [[histone]] modifications and [[heterochromatin]], and technical limitations affecting [[parthenogenesis]] and [[cloning]]. |
||
| − | ==The epigenome== |
||
| − | The '''epigenome''' is the overall epigenetic state of a cell. As one embryo can generate a multitude of cell fates during development, one genome could be said to give rise to many epigenomes. Taken to its extreme, this represents the total state of the cell, with the position of each molecule accounted for. |
||
| + | Epigenetic research uses a wide range of molecular biologic techniques to further our understanding of epigenetic phenomena, including [[chromatin immunoprecipitation]] (together with its large-scale variants [[ChIP-on-chip]] and [[ChIP-seq]]), [[fluorescent in situ hybridization]], methylation-sensitive [[restriction enzymes]], DNA adenine methyltransferase identification ([[DamID]]) and [[bisulfite sequencing]]. Furthermore, the use of [[bioinformatic]] methods is playing an increasing role ([[computational epigenetics]]). |
||
| − | ==Epigenetic inheritance== |
||
| − | ''Epigenetic inheritance'' is the transmission of information from a [[cell (biology)|cell]] or multicellular [[organism]] to its descendants without that information being encoded in the [[nucleotide]] sequence of the [[gene]]. |
||
| + | ==Etymology and definitions== |
||
| − | Epigenetic inheritance occurs in the development of multicellular organisms: dividing [[fibroblasts]] for instance give rise to new [[fibroblasts]] (rather than some other cell type) even though their genome is identical to that of all other cells. Quantitative genetic studies in [[mammal]]s and [[bird]]s can reveal [[maternal effect]]s, which is a form of epigenetic transmission, from one generation to the next. This was first observed in [[maize]]. Non-genetic paternal effects are rare. |
||
| + | The word "epigenetics" has been associated with many different definitions, and much of the confusion surrounding the use of the word "epigenetics" relates to the fact that it was originally defined to explain phenomena without knowing their molecular basis and with time became narrowly linked to certain phenomena as their molecular basis was discovered.<ref>Roloff, T.C., Nuber, U.A., 2005 Chromatin , epigenetics and stem cells. Eur J Cell Biol. 84, 123-135</ref> |
||
| + | The word "epigenetics" (as in "[[epigenetic landscape]]") was coined by [[Conrad Hal Waddington|C. H. Waddington]] in 1942 as a [[portmanteau]] of the words "[[genetics]]" and "[[Epigenesis (biology)|epigenesis]]".<ref name=waddington>{{cite journal|author=C.H. Waddington (1942)| title=The epigenotype| journal=Endeavour| volume=1| pages=18-20}}</ref> Epigenesis is an older word used to describe the differentiation of cells from a [[totipotent]] state in embryonic development (used in contrast to "preformationism"). At the time Waddington first used the term "epigenetics," the physical nature of genes and their role in heredity was not known. Epigenetics was Waddington's model of how genes within a multicellular organism interact with their surroundings to produce a [[phenotype]]. Because all cells within an organism inherit the same DNA sequences, [[cellular differentiation]] processes crucial for epigenesis rely strongly on epigenetic rather than genetic inheritance. [[Robin Holliday]] defined epigenetics as "the study of the mechanisms of temporal and spatial control of gene activity during the development of complex organisms."<ref>Holliday, R., 1990. Mechanisms for the control of gene activity during development. Biol. Rev. Cambr. Philos. Soc. 65, 431-471</ref> Thus, the word "epigenetic" can be used to describe any aspect other than DNA sequence that influences the development of an organism. |
||
| − | ===Heritable changes in gene function without DNA change=== |
||
| − | This includes the study of how environmental factors affecting a parent can result in changes in the way genes are expressed in the offspring {{ref|Waterland2003}}. |
||
| + | Another usage of the word "epigenetics" was employed by the psychologist [[Erik Erikson]], who developed an "epigenetic theory of human development," which focuses on psycho-social crises. |
||
| − | ===Processes involved in unfolding development of an organism=== |
||
| − | This includes [[gene regulation]] phenomena such as [[X-inactivation|X chromosome inactivation]] in [[mammal]]ian females, and [[gene silencing]] within an organism. |
||
| + | The modern usage of the word "epigenetic" is usually more narrow, referring to heritable traits (over rounds of cell division and sometimes transgenerationally) that do not involve changes to the underlying DNA sequence.<ref>Russo, V.E.A., Martienssen, R.A., Riggs, A.D., 1996 Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory Press, Plainview, NY.</ref> The Greek prefix "epi-" in the word "epigenetics" implies features that are "on top of" or "in addition to" genetics, and the current usage of the word reflects this—epigenetic traits exist on top of or in addition to the traditional molecular basis for inheritance. |
||
| − | ===Histone acetylation=== |
||
| − | One way the [[Gene expression|expression of a gene]] can be enhanced is through the [[acetylation]] on the K9 and K4 [[lysine]]s of the [[N-terminus]] tails of the internal [[histones]] of the [[nucleosome]]. Since lysine normally has a positive charge on the nitrogen at its end, it can bind the negatively charged phosphates of the DNA backbone and prevent them from repelling each other. When the charge is neutralized, the DNA can fold tightly, thus preventing access to the DNA by the [[transcription (genetics)|transcriptional]] machinery. When an acetyl group is added to the +NH2 of the lysine, it removes the positive charge and causes the DNA to repel itself and not fold up so tightly. When this occurs, complexes like SWI/SNF and other transcriptional factors can bind to the DNA, thus opening it up and exposing it to enzymes like [[RNA polymerase]] so transcription of the gene can occur. |
||
| + | The similarity of the word to "genetics" has generated many parallel usages. The "epigenome" is a parallel to the word "[[genome]]," and refers to the overall epigenetic state of a cell. The phrase "[[genetic code]]" has also been adapted—the "[[epigenetic code]]" has been used to describe the set of epigenetic features that create different phenotypes in different cells. Taken to its extreme, the "epigenetic code" could represent the total state of the cell, with the position of each molecule accounted for; more typically, the term is used in reference to systematic efforts to measure specific, relevant forms of epigenetic information such as the [[histone code hypothesis|histone code]] or [[DNA methylation]] patterns. |
||
| − | === Epigenetic inheritance systems === |
||
| + | ==Mechanisms== |
||
| − | Epigenetic inheritance systems (EISs) allow cells of different [[phenotype]] but identical [[genotype]] to transmit their phenotype to their offspring, even when the phenotype-inducing stimuli are absent, as is often the case. Three types of EISs may play a role in what has become known as cell memory {{Ref|Jablonka1992}}: |
||
| + | Several types of epigenetic inheritance systems may play a role in what has become known as cell memory:<ref name="jablonka92">{{cite journal |
||
| + | | last = Jablonka |
||
| + | | first = E |
||
| + | | coauthors = Lamb MJ and Lachmann M |
||
| + | | title = Evidence, mechanisms and models for the inheritance of acquired characteristics |
||
| + | | journal = J. Theoret. Biol. |
||
| + | | year = 1992 |
||
| + | | month = September |
||
| + | | volume = 158 |
||
| + | | issue = 2 |
||
| + | | pages = 245–268 |
||
| + | | url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WMD-4KFTYG9-8&_user=10&_handle=C-WA-A-E-E-MsSAYWW-UUW-U-U-E-U-U-AAZEEUZCDZ-AAZDVYDBDZ-ADYZVZWEA-E-U&_fmt=summary&_coverDate=09%2F21%2F1992&_rdoc=8&_orig=browse&_srch=%23toc%236932%231992%23998419997%23628456!&_cdi=6932&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=56159ca247a23a908a55cdabe8dd69e7}}</ref> |
||
| + | ===DNA methylation and chromatin remodeling=== |
||
| − | #''Steady-state systems.'' Some metabolic patterns are self-perpetuating. Sometimes a gene, after being turned on, transcribes a product (either directly or indirectly) that maintains the activity of that gene. Descendants of the cell in which the gene was turned on will inherit this activity, even if the original stimulus for gene-activation is no longer present. Also, diffusion of the gene's product to other cells can make the (heritable) characteristic spread. |
||
| + | [[Image:Nucleosome 1KX5 2.png|thumb|DNA associates with histone proteins to form chromatin.]] |
||
| − | #''[[Structural inheritance]] systems.'' In [[ciliate]]s such as ''[[Tetrahymena]]'' and ''[[Paramecium]]'', genetically identical cells show heritable differences in the patterns of ciliary rows on their cell surface. Experimentally altered patterns can be transmitted to daughter cells. It seems existing structures act as templates for new structures. The mechanisms of such inheritance are unclear, but reasons exist to assume that multicellular organisms also use existing cell structures to assemble new ones {{Ref|Tremblay1995}}. |
||
| + | Because the [[phenotype]] of a cell or individual is affected by which of its genes are transcribed, heritable [[Transcription (genetics)|transcription states]] can give rise to epigenetic effects. There are several layers of regulation of [[gene expression]], one of which is remodeling of chromatin, the complex of DNA and the [[histone]] proteins with which it associates. Chromatin remodeling is initiated by one of two things: |
||
| − | #''Chromatin-marking systems.'' [[Protein|Proteins]] or chemical groups that are attached to [[DNA]] and modify its activity are called ''chromatin marks''. These marks are copied with the [[DNA]]. For example, several [[cytosine|cytosines]] in [[eukaryote|eukaryotic]] [[DNA]] are methylated ([[5-methylcytosine]]). The number and pattern of such methylated [[cytosine|cytosines]] influences the functional state of the [[gene]]: low levels of methylation correspond to high potential activity while high levels correspond to low activity. While there are random changes in the methylation pattern, there are also very specific ones, induced by environmental factors. After [[DNA replication]], maintenance [[DNA methyltransferase]] make sure the methylation pattern of the parental [[DNA]] is copied to the daughter strand. |
||
| + | # [[posttranslational modification]] of the amino acids that make up histone proteins, |
||
| + | # or the addition of methyl groups to the DNA, at [[CpG site]]s, to convert cytosine to [[5-methylcytosine]]. |
||
| + | Whereas DNA is not completely stripped of [[nucleosome]]s during replication, it is possible that the remaining modified histones may act as templates, initiating identical modification of surrounding new histones after deposition. DNA methylation has a more clear method of propagation through the preferential methylation of hemimethylated symmetric sites by enzymes like Dnmt 1. |
||
| + | Although modifications occur throughout the histone sequence, the unstructured termini of histones (called histone tails) are particularly highly modified. These modifications include [[acetylation]], [[methylation]] and [[ubiquitylation]]. Acetylation is the most highly studied of these modifications. For example, acetylation of the K14 and K9 [[lysine]]s of the tail of histone H3 by histone acetyltransferase enzymes (HATs) is generally correlated with transcriptional competence. |
||
| − | == Comparison to standard theories == |
||
| + | |||
| − | Epigenetic variants exhibit spontaneous emergence and reversion. However, they can be induced by the presence of other genetic factors, and some alleles of a gene have been shown to convert the epigenetic status of the same locus on the homologous chromosome. Environmental factors are also known to influence the emergence and reversion of epigenetic factors. This produces the possibility that epigenetic variations might be produced at several loci and in several cells or organisms. |
||
| + | One mode of thinking is that this tendency of acetylation to be associated with "active" transcription is biophysical in nature. Because lysine normally has a positive charge on the nitrogen at its end, lysine can bind the negatively charged phosphates of the DNA backbone and prevent them from repelling each other. The acetylation event converts the positively charged amine group on the side chain into a neutral amide linkage. This removes the positive charge causing the DNA to repel itself. When this occurs, complexes like SWI/SNF and other transcriptional factors can bind to the DNA, thus opening it up and exposing it to enzymes like [[RNA polymerase]] so transcription of the gene can occur. |
||
| + | In addition, the positively charged tails of histone proteins from one nucleosome may interact with the histone proteins on a neighboring nucleosome, causing them to pack closely. Lysine acetylation may interfere with these interactions, causing the chromatin structure to open up. |
||
| − | ===Epigenetic coding and evolution=== |
||
| − | One question which is now raised is to what extent does epigenetic inheritance play a direct role in [[evolution]]? Since the discovery of the structure of DNA in the mid-20th century, biologists have held that the only role the environment plays is in the phase of selection: the environment determines on what grounds selection takes place and what characteristics are necessary for better reproduction opportunities. |
||
| + | Lysine acetylation may also act as a beacon to recruit other activating chromatin modifying enzymes (and basal transcription machinery as well). Indeed, the bromodomain—a protein segment (domain) that specifically binds acetyl-lysine—is found in many enzymes that help activate transcription including the SWI/SNF complex (on the protein polybromo). It may be that acetylation acts in this and the previous way to aid in transcriptional activation. |
||
| − | For selection to be possible, individuals within a species must differ somewhat. Genes that provide characteristics that allow an organism to survive in its environment become more common over time, while genes that provide characteristics that make the organism less likely to survive become less common over time. |
||
| + | The idea that modifications act as docking modules for related factors is borne out by histone methylation as well. Methylation of lysine 9 of histone H3 has long been associated with constitutively transcriptionally silent chromatin (constitutive [[heterochromatin]]). It has been determined that a chromodomain (a domain that specifically binds methyl-lysine) in the transcriptionally repressive protein [[Heterochromatin Protein 1|HP1]] recruits HP1 to K9 methylated regions. One example that seems to refute the biophysical model for acetylation is that tri-methylation of histone H3 at lysine 4 is strongly associated with (and required for full) transcriptional activation. Tri-methylation in this case would introduce a fixed positive charge on the tail. |
||
| − | These genetic differences between individuals are thought to arise from random mutations, and in organisms that reproduce sexually, from [[meiosis]]. These differences physically exist as changes in the nucleotide base sequence of [[DNA]]. The environment can influence these variations. For example, [[radioactivity]] randomly changes the base sequence of [[DNA]]. |
||
| + | It should be emphasized that differing histone modifications are likely to function in differing ways; acetylation at one position is likely to function differently than acetylation at another position. Also, multiple modifications may occur at the same time, and these modifications may work together to change the behavior of the nucleosome. The idea that multiple dynamic modifications regulate gene transcription in a systematic and reproducible way is called the [[histone code]]. |
||
| − | Some forms of epigenetic inheritance may be maintained even through the production of germ cells ([[meiosis]]). |
||
| + | DNA methylation frequently occurs in repeated sequences, and may help to suppress '[[junk DNA]]':<ref name="chedin92">{{cite web |
||
| − | A number of experimental studies seem to indicate that epigenetic inheritance plays a part in the evolution of complex organisms. For example, Tremblay et al. {{Ref|Tremblay1995}}, have shown that methylation differences between maternally and paternally inherited alleles of the mouse H19 gene are preserved. There are also numerous reports of heritable epigenetic marks in plants. |
||
| + | | last = Chédin |
||
| + | | first = F |
||
| + | | title = The Chedin Laboratory |
||
| + | | year = 1992 |
||
| + | | url = http://www.mcb.ucdavis.edu/faculty-labs/chedin/research.htm |
||
| + | | accessdate=2006-12-28}}</ref> Because [[5-methylcytosine]] is chemically very similar to [[thymidine]], CpG sites are frequently mutated and become rare in the genome, except at [[CpG islands]] where they remain unmethylated. Epigenetic changes of this type thus have the potential to direct increased frequencies of permanent genetic mutation. [[DNA methylation]] patterns are known to be established and modified in response to environmental factors by a complex interplay of at least three independent [[DNA methyltransferase]]s, DNMT1, DNMT3A and DNMT3B, the loss of any of which is lethal in mice.<ref name="li92">{{cite journal |
||
| + | | last = Li |
||
| + | | first = E |
||
| + | | coauthors = Bestor TH and Jaenisch R |
||
| + | | title = Targeted mutation of the DNA methyltransferase gene results in embryonic lethality |
||
| + | | journal = Cell |
||
| + | | year = 1992 |
||
| + | | month = June |
||
| + | | volume = 69 |
||
| + | | issue = 6 |
||
| + | | pages = 915–926 |
||
| + | | url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WSN-4D57093-1R&_coverDate=06%2F12%2F1992&_alid=515191593&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=7051&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=19d33f62482a44052266e684682a06da}}</ref> DNMT1 is the most abundant methyltransferase in somatic cells,<ref name="robertson99">{{cite journal |
||
| + | | last = Robertson |
||
| + | | first = KD |
||
| + | | coauthors = Uzyolgi E, Lian G et al |
||
| + | | title = The human DNA methyltransferases (DNMTs) 1, 3a, 3b: Coordinate mRNA expression in normal tissues and overexpression in tumors |
||
| + | | journal = Nucleic Acids Res |
||
| + | | year = 1999 |
||
| + | | month = June |
||
| + | | volume = 27 |
||
| + | | issue = 11 |
||
| + | | pages = 2291–2298 |
||
| + | | url = http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=10325416}}</ref> localizes to replication foci,<ref name="leonhardt92">{{cite journal |
||
| + | | last = Leonhardt |
||
| + | | first = H |
||
| + | | coauthors = Page AW, Weier HU, Bestor TH |
||
| + | | title = A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei |
||
| + | | journal = Cell |
||
| + | | year = 1992 |
||
| + | | month = November |
||
| + | | volume = 71 |
||
| + | | issue = 5 |
||
| + | | pages = 865–873 |
||
| + | | url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WSN-4D0YB27-19&_coverDate=11%2F27%2F1992&_alid=515194074&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=7051&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=9bdfefee567ff4ea6226007214ffbc34}}</ref> has a 10–40-fold preference for hemimethylated DNA and interacts with the proliferating cell nuclear antigen (PCNA).<ref name="chuang97">{{cite journal |
||
| + | | last = Chuang |
||
| + | | first = LS |
||
| + | | coauthors = Ian HI, Koh TW et al |
||
| + | | title = Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1 |
||
| + | | journal = Science |
||
| + | | year = 1997 |
||
| + | | month = September |
||
| + | | volume = 277 |
||
| + | | issue = 5334 |
||
| + | | pages = 1996–2000 |
||
| + | | url = http://www.sciencemag.org/cgi/content/abstract/277/5334/1996}}</ref> By preferentially modifying hemimethylated DNA, DNMT1 transfers patterns of methylation to a newly synthesized strand after [[DNA replication]], and therefore is often referred to as the ‘maintenance' methyltransferase.<ref name="robertson00">{{cite journal |
||
| + | | last = Robertson |
||
| + | | first = KD |
||
| + | | coauthors = Wolffe AP |
||
| + | | title =DNA methylation in health and disease |
||
| + | | journal = Nat Rev Genet |
||
| + | | year = 2000 |
||
| + | | month = October |
||
| + | | volume = 1 |
||
| + | | issue = 1 |
||
| + | | pages = 11–19 |
||
| + | | url = http://www.nature.com/nrg/journal/v1/n1/abs/nrg1000_011a_fs.html}}</ref> DNMT1 is essential for proper embryonic development, imprinting and X-inactivation.<ref name="li92" /><ref name="li93">{{cite journal |
||
| + | | last = Li |
||
| + | | first = E |
||
| + | | coauthors = Beard C and Jaenisch R |
||
| + | | title =Role for DNA methylation in genomic imprinting |
||
| + | | journal = Nature |
||
| + | | year = 1993 |
||
| + | | month = December |
||
| + | | volume = 366 |
||
| + | | issue = 6453 |
||
| + | | pages = 362–365 |
||
| + | | url = http://www.nature.com/nature/journal/v366/n6453/abs/366362a0.html}}</ref> |
||
| + | Chromosomal regions can adopt stable and heritable alternative states resulting in bistable gene expression without changes to the DNA sequence. Epigenetic control is often associated with alternative covalent modifications of histones. The stability and heritability of states of larger chromosomal regions are often thought to involve positive feedback where modified nucleosomes recruit enzymes that similarly modify nearby nucleosomes. A simplified stochastic model for this type of epigenetics is found |
||
| − | That epigenetic heredity seems to exist transgenerationally in complex organisms can be explained by allowing for minor epigenetic changes not affecting [[totipotency]]. This puts some constraints on the extent to which epigenetic changes can be brought upon [[DNA]], but it allows for EISs to play direct evolutionary roles. |
||
| + | [http://cmol.nbi.dk/models/epigen/Epigen.html here] |
||
| + | <ref> |
||
| + | I.B. Dodd, M.A. Micheelsen, K. Sneppen and G. Thon (2007). |
||
| + | Theoretical Analysis of Epigenetic Cell Memory by Nucleosome Modification |
||
| + | ''Cell'' '''129''':813-822.</ref> |
||
| + | . |
||
| + | Because DNA methylation and chromatin remodeling play such a central role in many types of epigenic inheritance, the word "epigenetics" is sometimes used as a synonym for these processes. However, this can be misleading. Chromatin remodeling is not always inherited, and not all epigenetic inheritance involves chromatin remodeling.<ref>Mark Ptashne, 2007. On the use of the word ‘epigenetic’. ''Current Biology'', '''17'''(7):R233-R236. {{doi|10.1016/j.cub.2007.02.030}}</ref> |
||
| − | However, in none of these cases does a cell reprogram its DNA to produce genes that increase its ability to survive in a given environment. |
||
| + | It has been suggested that the [[histone code]] could be mediated by the effect of small RNAs. The recent discovery and characterization of a vast array of small (21- to 26-nt), non-coding RNAs suggests that there is an RNA component, possibly involved in epigenetic gene regulation. Small interfering RNAs can modulate transcriptional gene expression via epigenetic modulation of targeted [[promoter]]s.<ref name=Morris>{{cite book |chapterurl=http://www.horizonpress.com/rnareg|author= Morris KV|year=2008|chapter=Epigenetic Regulation of Gene Expression|title=RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity|publisher=Caister Academic Press|id=[http://www.horizonpress.com/rnareg ISBN 978-1-904455-25-7]}}</ref> |
||
| − | ===Possible epigenetic effects in humans=== |
||
| + | ===RNA transcripts and their encoded proteins=== |
||
| − | Work by [http://www.alspac.bris.ac.uk/welcome/marcus_biog.shtml Marcus Pembrey] indicates that both [[Angelman syndrome]] and [[Prader-Willi syndrome]] appear to be produced by the same genetic mutation, [[chromosome 15q partial deletion]], and that the particular syndrome that a child has seems to depend on whether the mutation was inherited from the child's mother or father. This suggests that inherited aspects of development may depend on more than just the "conventional" genome. |
||
| + | Sometimes a gene, after being turned on, transcribes a product that (either directly or indirectly) maintains the activity of that gene. For example, [[Hnf4]] and [[MyoD]] enhance the transcription of many liver- and muscle-specific genes, respectively, including their own, through the [[transcription factor]] activity of the [[proteins]] they encode. Other epigenetic changes are mediated by the production of different [[Splicing (genetics)|splice forms]] of [[RNA]], or by formation of double-stranded RNA ([[RNAi]]). Descendants of the cell in which the gene was turned on will inherit this activity, even if the original stimulus for gene-activation is no longer present. These genes are most often turned on or off by [[signal transduction]], although in some systems where [[syncytia]] or [[gap junctions]] are important, RNA may spread directly to other cells or nuclei by [[diffusion]]. A large amount of RNA and protein is contributed to the [[zygote]] by the mother during [[oogenesis]] or via [[nurse cell]]s, resulting in [[maternal effect]] phenotypes. A smaller quantity of sperm RNA is transmitted from the father, but there is recent evidence that this epigenetic information can lead to visible changes in several generations of offspring.<ref name="choi06">{{cite web |
||
| + | | author= Choi CQ |
||
| + | | title=The Scientist: RNA can be hereditary molecule |
||
| + | | publisher = The Scientist |
||
| + | | url=http://www.the-scientist.com/news/display/23494 |
||
| + | | date=2006-05-25| accessdate=2006}}</ref> |
||
| + | ===Prions=== |
||
| + | {{details|Prions}} |
||
| + | [[Prion]]s are infectious forms of [[protein]]s. Proteins generally fold into discrete units which perform distinct cellular functions, but some proteins are also capable of forming an infectious conformational state known as a prion. Although often viewed in the context of [[Transmissible spongiform encephalopathy|infectious disease]], prions are more loosely defined by their ability to catalytically convert other native state versions of the same protein to an infectious conformational state. It is in this latter sense that they can be viewed as epigenetic agents capable of inducing a phenotypic change without a modification of the genome.<ref>{{cite journal| title=Epigenetic inheritance and prions| author=A. Yool and W.J. Edmunds| journal=Journal of Evolutionary Biology| year=1998| pages=241-242| volume=11}}</ref> |
||
| + | [[Fungal prion]]s are considered epigenetic because the infectious phenotype caused by the prion can be inherited without modification of the genome. [[PSI (prion)|PSI+]] and URE3, discovered in [[Saccharomyces cerevisiae|yeast]] in 1965 and 1971, are the two best studied of this type of prion.<ref>{{cite journal|title=[PSI], a cytoplasmic suppressor of super-suppression in yeast| author=B.S. Cox| journal=Heredity| volume=20| pages=505-521| year=1965}}</ref><ref>{{cite journal|title=Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast| author=F. Lacroute| journal=Journal of Bacteriology| volume=106| pages=519-522| year=1971}}</ref> Prions can have a phenotypic effect through the sequestration of protein in aggregates, thereby reducing that protein's activity. In PSI+ cells, the loss of the Sup35 protein (which is involved in termination of translation) causes ribosomes to have a higher rate of read-through of stop codons, an effect which results in suppression of [[nonsense mutation]]s in other genes.<ref>{{cite journal|title=Extrachromosomal psi+ determinant suppresses nonsense mutations in yeast|author=S.W. Liebman and F. Sherman| journal=Journal of Bacteriology| year=1979| volume=139| issue=3| pages=1068-1071}} [http://jb.asm.org/cgi/content/abstract/139/3/1068 Free full text available]</ref> The ability of Sup35 to form prions may be a conserved trait. It could confer an adaptive advantage by giving cells the ability to switch into a PSI+ state and express dormant genetic features normally terminated by premature stop codon mutations.<ref>{{cite journal| title=A yeast prion provides a mechanism for genetic variation and phenotypic diversity| author=H.L. True and S.L. Lindquist| journal=Nature| year=2000| volume=407| pages=477-483}}</ref><ref>{{cite journal| title=Prions as adaptive conduits of memory and inheritance| author=J. Shorter and S. Lindquist| journal=Nature Reviews Genetics| volume=6| issue=6| pages=435-450| year=2005}}</ref> |
||
| − | == Historical notes == |
||
| + | ===Structural inheritance systems=== |
||
| − | Some biologists at one time believed that genetics, which seemed to postulate a one-to-one correspondence between [[genotype]] and [[phenotype]], could not explain [[cell differentiation]]. They developed a theory that each undifferentiated cell underwent a crisis that determined its fate, which was not inherent in its genes, and was therefore (borrowing from the Greek) epigenetic. |
||
| + | {{details|Structural inheritance}} |
||
| + | In [[ciliate]]s such as ''[[Tetrahymena]]'' and ''[[Paramecium]]'', genetically identical cells show heritable differences in the patterns of ciliary rows on their cell surface. Experimentally altered patterns can be transmitted to daughter cells. It seems existing structures act as templates for new structures. The mechanisms of such inheritance are unclear, but reasons exist to assume that multicellular organisms also use existing cell structures to assemble new ones.<ref>{{cite book |title=Cycles of Contingency: Developmental Systems and Evolution |last=Oyama |first=Susan |coauthors=Paul E. Griffiths, Russell D. Gray |year=2001 |publisher=MIT Press |isbn=0262650630 }}</ref> |
||
| − | The psychologist [[Erik Erikson]] developed an epigenetic theory of human development which focuses on psycho-social crises. |
||
| − | In Erikson's view, each individual goes through several developmental stages, the transition between each of which is marked by a crisis. According to the theory, although the stages are largely predetermined by genetics, the manner in which the crises are resolved is not; by analogy with the epigenetic theory of cell differentiation, the process was said to be epigenetic. |
||
| + | ==Functions and consequences== |
||
| − | The biologist [[C.H. Waddington]] is sometimes credited with coining the term epigenetics in 1942, when he defined it as “the branch of biology which studies the causal interactions between genes and their products which bring the phenotype into being”. However the term "epigenesis" goes back at least to 1896 (see References). |
||
| + | ===Development=== |
||
| + | Somatic epigenetic inheritance, particularly through DNA methylation and chromatin remodeling, is very important in the development of multicellular eukaryotic organisms. The genome sequence is static (with some notable exceptions), but cells differentiate in many different types, which perform different functions, and respond differently to the environment and intercellular signalling. Thus, as individuals develop, [[morphogen]]s activate or silence genes in an epigenetically heritable fashion, giving cells a "memory". In mammals, most cells terminally differentiate, with only [[stem cells]] retaining the ability to differentiate into several cell types ("totipotency" and "multipotency"). In mammals, some stem cells continue producing new differentiated cells throughout life, but mammals are not able to respond to loss of some tissues, for example, the inability to regenerate limbs, which some other animals are capable of. Unlike animals, plant cells do not terminally differentiate, remaining totipotent with the ability to give rise to a new individual plant. While plants do utilise many of the same epigenetic mechanisms as animals, such as chromatin remodeling, it has been hypothesised that plant cells do not have "memories", resetting their gene expression patterns at each cell division using positional information from the environment and surrounding cells to determine their fate.<ref>Silvia Costa and Peter Shaw. 2006. 'Open Minded' cells: how cells can change fate. ''Trends in Cell Biology'' '''17'''(3):101-106. {{doi|10.1016/j.tcb.2006.12.005}}</ref> |
||
| + | ===Medicine=== |
||
| − | [[Epigenetic inheritance]] is the transmission of information from a cell or multicellular organism to its descendants without that information being encoded in the nucleotide sequence of the gene. This information, rather, can be stored as [[methylation]] on a nucleotide base, without changing the base sequence. The study of epigenetic inheritance is known as epigenetics. |
||
| + | Epigenetics has many and varied potential medical applications. Congenital genetic disease is well understood, and it is also clear that epigenetics can play a role, for example, in the case of [[Angelman syndrome]] and [[Prader-Willi syndrome]]. These are normal genetic diseases caused by gene deletions, but are unusually common because individuals are essentially [[hemizygous]] because of [[Genomic Imprinting|genomic imprinting]], and therefore a single gene knock out is sufficient to cause the disease, where most cases would require both copies to be knocked out.<ref>{{OMIM|105830}}</ref> |
||
| − | === |
+ | ===Evolution=== |
| + | Although epigenetics in multicellular organisms is generally thought to be a mechanism involved in differentiation, with epigenetic patterns "reset" when organisms reproduce, there have been some observations of transgenerational epigenetic inheritance (e.g., the phenomenon of [[paramutation]] observed in maize). Although most of these multigenerational epigenetic traits are gradually lost over several generations, the possibility remains that multigenerational epigenetics could be another aspect to evolution and adaptation. These effects may require enhancements to the standard conceptual framework of the [[modern evolutionary synthesis]].<ref>{{cite book |first=Eva |last=Jablonka |authorlink=Eva Jablonka |coauthors=Marion J. Lamb |title=Evolution in Four Dimensions |publisher=MIT Press |year=2005 |id=ISBN 0-262-10107-6}}</ref><ref>See also [[Denis Noble]] ''The Music of Life'' see esp pp93-8 and p48 where he cites Jablonka & Lamb and [[Massimo Pigliucci]]'s review of Jablonka and Lamb in [[Nature (journal)|Nature]] '''435''', 565-566 (2 June 2005)</ref> |
||
| − | The term '''''epigenetics''''' has over time been used in various senses, in part because the Greek prefix ''epi-'' has at least six meanings in English (including 'on', 'after' and 'in addition'), but also because various theories of epigenetic development, inheritance, and evolution have been proposed. |
||
| + | |||
| + | Epigenetic features may play a role in short-term adaptation of species by allowing for reversible phenotype variability. The modification of epigenetic features associated with a region of DNA allows organisms, on a multigenerational time scale, to switch between phenotypes that express and repress that particular gene.<ref name=rando_and_verstrepen>{{cite journal| author=O.J. Rando and K.J. Verstrepen| title=Timescales of Genetic and Epigenetic Inheritance| journal=Cell| volume=128| pages=655-668| year=2007}}</ref> Whereas the DNA sequence of the region is not mutated, this change is reversible. It has also been speculated that organisms may take advantage of differential mutation rates associated with epigenetic features to control the mutation rates of particular genes.<ref name=rando_and_verstrepen /> |
||
| + | |||
| + | Epigenetic changes have also been observed to occur in response to environmental exposure—for example, mice given some dietary supplements have epigenetic changes affecting expression of the [[agouti gene]], which affects their fur color, weight, and propensity to develop cancer.<ref>{{cite journal| author=Cooney, CA, Dave, AA, and Wolff, GL| title=Maternal Methyl Supplements in Mice Affect Epigenetic Variation and DNA Methylation of Offspring| year=2002| journal=Journal of Nutrition| volume=132| pages=2393S-2400S}}[http://jn.nutrition.org/cgi/content/full/132/8/2393S available online]</ref><ref name="waterland">{{cite journal| author= Waterland RA and Jirtle RL| title = Transposable elements: Targets for early nutritional effects on epigenetic gene regulation| journal = Molecular and Cellular Biology| year = 2003| month = August| volume = 23| issue = 15| pages = 5293-5300| url = http://mcb.asm.org/cgi/content/full/23/15/5293}}</ref> |
||
| + | Although there is no reason to suspect that such a process is anything other than a product of genetics, and therefore orthodox evolutionary mechanisms, the observation of trans-generational epigenetic change occurring in response to environmental factors is reminiscent of the 19th century [[Lamarckism|Lamarckian hypothesis]] of inheritance and evolution. |
||
| + | |||
| + | ==Epigenetic effects in humans== |
||
| + | === Genomic imprinting and related disorders === |
||
| + | Some human disorders are associated with genomic imprinting, a phenomenon in mammals where the father and mother contribute different epigenetic patterns for specific genomic loci in their germ cells.<ref>{{cite journal| title=Genomic imprinting in mammals: Emerging themes and established theories| author=A.J. Wood and A.J. Oakey| journal=PLOS Genetics| volume=2| issue=11| year=2006| pages=1677-1685}} [http://genetics.plosjournals.org/perlserv/?request=get-document&doi=10.1371%2Fjournal.pgen.0020147 available online]</ref> The most well-known case of imprinting in human disorders is that of [[Angelman syndrome]] and [[Prader-Willi syndrome]]—both can be produced by the same genetic mutation, [[chromosome 15q partial deletion]], and the particular syndrome that will develop depends on whether the mutation is inherited from the child's mother or from their father.<ref>{{cite journal| title=Angelman and Prader-Willi syndromes share a common chromosome deletion but differ in parental origin of the deletion| author=J.H.M. Knoll, R.D. Nicholls, R.E. Magenis, J.M. Graham Jr, M. Lalande, S.A. Latt| journal=American Journal of Medical Genetics| volume=32| pages=285-290| year=1989}}</ref> This is due to the presence of [[Genomic Imprinting|genomic imprinting]] in the region. [[Beckwith-Wiedemann syndrome]] is also associated with genomic imprinting, often caused by abnormalities in maternal genomic imprinting of a region on chromosome 11. |
||
| + | |||
| + | ===Transgenerational epigenetic observations=== |
||
| + | Marcus Pembrey and colleagues also observed that the paternal (but not maternal) grandsons of Swedish boys who were exposed to famine in the 19th century were less likely to die of cardiovascular disease; if food was plentiful then [[diabetes]] mortality in the grandchildren increased, suggesting that this was a transgenerational epigenetic inheritance.<ref>Pembrey ME, Bygren LO, Kaati G, ''et al''. Sex-specific, male-line transgenerational responses in humans. ''Eur J Hum Genet'' 2006; 14: 159-66. PMID 16391557. [[Robert Winston]] refers to this study in a [http://www.dundee.ac.uk/externalrelations/events/lectures.html lecture]; see also discussion at [[Leeds University]], [http://www.fbs.leeds.ac.uk/staff/pm/epigenetics.htm#exciting2 here]</ref> |
||
| + | |||
| + | === Cancer and developmental abnormalities === |
||
| + | A variety of compounds are considered as epigenetic [[carcinogens]]—they result in an increased incidence of tumors, but they do not show [[mutagenic|mutagen]] activity (toxic compounds or pathogens that cause tumors incident to increased regeneration should also be excluded). Examples include [[diethylstilbestrol]], [[arsenite]], [[hexachlorobenzene]], and [[nickel]] compounds. |
||
| + | |||
| + | Many teratogens exert specific effects on the fetus by epigenetic mechanisms.<ref name="bishop97">{{cite journal |
||
| + | | last = Bishop |
||
| + | | first = JB |
||
| + | | coauthors = Witt KL and Sloane RA |
||
| + | | title = Genetic toxiticities of human teratogens |
||
| + | | journal = Mutat Res |
||
| + | | year = 1997 |
||
| + | | month = December |
||
| + | | volume = 396 |
||
| + | | issue = 1-2 |
||
| + | | pages = 9–43 |
||
| + | | url = http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T2C-3TGW0WR-19&_coverDate=12%2F12%2F1997&_alid=515200131&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=4915&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=991625903beeedc77a9455d6fa2382a9}}</ref><ref name="gurvich04">{{cite journal |
||
| + | | last = Gurvich |
||
| + | | first = N |
||
| + | | coauthors = Berman MG, Wittner BS et al |
||
| + | | title = Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo |
||
| + | | journal = FASEB J |
||
| + | | year = 2004 |
||
| + | | month = July |
||
| + | | volume = 19 |
||
| + | | issue = 9 |
||
| + | | pages = 1166–1168 |
||
| + | | url = http://www.fasebj.org/cgi/reprint/04-3425fjev1}}</ref> While epigenetic effects may preserve the effect of a teratogen such as [[diethylstilbestrol]] throughout the life of an affected child, the possibility of birth defects resulting from exposure of fathers or in second and succeeding generations of offspring has generally been rejected on theoretical grounds and for lack of evidence.<ref name="smithells98">{{cite journal |
||
| + | | last = Smithells |
||
| + | | first = D |title = Does thalidomide cause second generation birth defects? |
||
| + | | journal = Drug Saf |
||
| + | | year = 1998 |
||
| + | | month = November |
||
| + | | volume = 19 |
||
| + | | issue = 5 |
||
| + | | pages = 339–341 |
||
| + | | url = http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=9825947}}</ref> However, a range of male-mediated abnormalities have been demonstrated, and more are likely to exist.<ref name="friedler96">{{cite journal |
||
| + | | last = Friedler |
||
| + | | first = G |
||
| + | | title = Paternal exposures: impact on reproductive and developmental outcome. An overview. |
||
| + | | journal = Pharmacol Biochem Behav |
||
| + | | year = 1996 |
||
| + | | month = December |
||
| + | | volume = 55 |
||
| + | | issue = 4 |
||
| + | | pages = 691–700 |
||
| + | | url = http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=8981601}}</ref> [http://www.fda.gov/cder/foi/label/2004/050794lbl.pdf FDA label information] for Vidaza(tm), a formulation of [[5-azacytidine|5-azacitidine]] (an unmethylatable analog of cytidine that causes hypomethylation when incorporated into DNA) states that "men should be advised not to father a child" while using the drug, citing evidence in treated male mice of reduced fertility, increased embryo loss, and abnormal embryo development. In rats, endocrine differences were observed in offspring of males exposed to morphine.<ref name="cicero91">{{cite journal |
||
| + | | last = Cicero |
||
| + | | first = TJ |
||
| + | | coauthors = Adams NL, Giodarno A et al |
||
| + | | title = Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring |
||
| + | | journal = J Pharmacol Exp Ther. |
||
| + | | year = 1991 |
||
| + | | month = March |
||
| + | | volume = 256 |
||
| + | | issue = 3 |
||
| + | | pages = 1086–1093 |
||
| + | | url = http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=2005573}}</ref> In mice, second generation effects of diethylstilbesterol have been described occurring by epigenetic mechanisms.<ref name="newbold06">{{cite journal |
||
| + | | last = Newbold |
||
| + | | first = RR |
||
| + | | coauthors = Padilla-Banks E and Jefferson WN |
||
| + | | title = Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations |
||
| + | | journal = Endocrinology |
||
| + | | year = 2006 |
||
| + | | month = June |
||
| + | | volume = 147 |
||
| + | | issue = 6 Suppl |
||
| + | | pages = S11–S17 |
||
| + | | url = http://endo.endojournals.org/cgi/content/abstract/147/6/s11}}</ref> |
||
| + | |||
| + | == Epigenetics in microorganisms == |
||
| + | Bacteria make widespread use of postreplicative DNA methylation for the epigenetic control of DNA-protein interactions. Bacteria make use of DNA adenine methylation (rather than DNA cytosine methylation) as an epigenetic signal. DNA adenine methylation is important in bacteria virulence in organisms such as ''[[Escherichia coli]]'', ''[[Salmonella]], [[Vibrio]], [[Yersinia]], [[Haemophilus]]'', and ''[[Brucella]]''. In ''[[Alphaproteobacteria]]'', methylation of adenine regulates the cell cycle and couples gene transcription to DNA replication. In ''[[Gammaproteobacteria]]'', adenine methylation provides signals for DNA replication, chromosome segregation, mismatch repair, packaging of bacteriophage, transposase activity and regulation of gene expression.<ref name="Casadesus">{{cite journal| author= Casadesus J and Low D| title = Epigenetic Gene Regulation in the Bacterial World| journal = Microbiol Mol Biol Rev| year = 2006| month = September| volume = 70| issue = 3| pages = 830-856}}</ref> <ref name=JorgTost>{{cite book | author = Tost J (editor). | title = Epigenetics | publisher = Caister Academic Press | year = 2008 | url=http://www.horizonpress.com/epi | id = [http://www.horizonpress.com/epi ISBN 978-1-904455-23-3 ]}}</ref> |
||
| + | |||
| + | The [[yeast]] [[prion]] PSI is generated by a conformational change of a translation termination factor, which is then inherited by daughter cells. This can provide a survival advantage under adverse conditions. This is an example of epigenetic regulation enabling unicellular organisms to respond rapidly to environmental stress. Prions can be viewed as epigenetic agents capable of inducing a phenotypic change without modification of the genome.<ref name=JorgTost>{{cite book | author = Tost J (editor). | title = Epigenetics | publisher = Caister Academic Press | year = 2008 | url=http://www.horizonpress.com/epi | id = [http://www.horizonpress.com/epi ISBN 978-1-904455-23-3 ]}}</ref> |
||
== See also == |
== See also == |
||
| − | *[[ |
+ | * [[Histone code]] |
| − | *[[ |
+ | * [[Baldwinian evolution]] |
| + | * [[Barbara McClintock]] |
||
| − | *[[Evolutionary developmental psychology]] |
||
| − | *[[Molecular biology]] |
||
* [[Centromere]] |
* [[Centromere]] |
||
| + | * [[Evolutionary developmental psychology]] |
||
| − | * [[Imprinting (genetics)|Imprinting]] |
||
| − | * [[ |
+ | * [[Molecular biology]] |
| − | * [[ |
+ | * [[Somatic epitype]] |
| − | * [[Prion]] |
||
* [[Weismann barrier]] |
* [[Weismann barrier]] |
||
| + | == Further reading == |
||
| − | ==External links== |
||
| + | <!-- Dead note "Jablonka2002": Eva Jablonka and Marion J. Lamb. The Changing Concept of Epigenetics. ''Annals of the New York Academy of Sciences'' '''981''':82-96 (2002). --> |
||
| − | * [http://www.epigenome-noe.net/index.php The Epigenome Network of Excellence (NoE)] |
||
| − | * [http://www.epigenome.org/ Human Epigenome Consortium] |
||
| − | * [http://www.bbc.co.uk/sn/tvradio/programmes/horizon/ghostgenes.shtml BBC Article on Epigenetics] |
||
| − | |||
| − | == References == |
||
| − | *{{Note|Waterland2003}} R.A. Waterland, R.L. Jirtle, "Transposable elements: Targets for early nutritional effects on epigenetic gene regulation", ''Molecular and Cellular Biology'' [[2003]] August 1;'''23'''(15):5293-5300. |
||
| − | *{{Note|Jablonka1992}} E. Jablonka; M. Lachmann and M.J. Lamb; ''Evidence, mechanisms and models for the inheritance of acquired characteristics'', J. Theoret. Biol. 158: 245-268 (1992) |
||
| − | *{{Note|Tremblay1995}} K.D. Tremblay; J.R. Saam; R.S. Ingram; S.M. Tilghman and M.S. Bartolomei; ''A paternal-specific methylation imprint marks the alleles of the mouse H19 gene.'' Nature Genet. 9: 407-413 (1995) |
||
*[[Oskar Hertwig]], 1849-1922. ''Biological problem of today: preformation or epigenesis? The basis of a theory of organic development''. W. Heinemann: London, [[1896]]. |
*[[Oskar Hertwig]], 1849-1922. ''Biological problem of today: preformation or epigenesis? The basis of a theory of organic development''. W. Heinemann: London, [[1896]]. |
||
| − | *{{Note|Jablonka2002}} Eva Jablonka and Marion J. Lamb. The Changing Concept of Epigenetics. ''Annals of the New York Academy of Sciences'' '''981''':82-96 (2002). |
||
* R. Jaenisch and A. Bird (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. ''Nat. Genet.'' '''33''' (Suppl) 245-254. |
* R. Jaenisch and A. Bird (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. ''Nat. Genet.'' '''33''' (Suppl) 245-254. |
||
*[[Joshua Lederberg]], "The Meaning of Epigenetics", ''The Scientist'' '''15'''(18):6, Sep. 17, [[2001]]. |
*[[Joshua Lederberg]], "The Meaning of Epigenetics", ''The Scientist'' '''15'''(18):6, Sep. 17, [[2001]]. |
||
| Line 96: | Line 271: | ||
* B. D. Strahl and C. D. Allis (2000) The language of covalent histone modifications. ''Nature'' '''403''', 41-45. |
* B. D. Strahl and C. D. Allis (2000) The language of covalent histone modifications. ''Nature'' '''403''', 41-45. |
||
*[[C.H. Waddington]] ([[1942]]), "The epigenotype". ''Endeavour'' '''1''', 18–20. |
*[[C.H. Waddington]] ([[1942]]), "The epigenotype". ''Endeavour'' '''1''', 18–20. |
||
| + | *B. McClintock (1978) Mechanisms that Rapidly Reorganize the Genome. ''Stadler Symposium'' vol 10:25-48 |
||
| − | *R.A. Waterland, R.L. Jirtle, "Transposable elements: Targets for early nutritional effects on epigenetic gene regulation", ''Molecular and Cellular Biology'' [[2003]] August 1;'''23'''(15):5293-5300. |
||
| − | *G.W. Grimes; K.J. Aufderheide; |
+ | *G.W. Grimes; K.J. Aufderheide; ''Cellular Aspects of Pattern Formation: the Problem of Assembly.'' Monographs in Developmental Biology, Vol. 22. Karger, Basel (1991) |
| + | * [[Eva Jablonka]] and [[Marion J. Lamb]] ''Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life'' The MIT Press (2005) ISBN 978-0262101073 |
||
| + | * [http://www.uq.edu.au/biohumanities/webpdfs/moleculardevelop.pdf Article on The Philosophy of Molecular and Developmental Biology] to appear in Blackwell’s Guide to Philosophy of Science,. P.K. Machamer and M. Silberstein (Eds). |
||
| + | *[[Epigenetics]] edited by C. David Allis, Thomas Jenuwein, Danny Reinberg, and Marie-Laure Caparros. Cold Spring Harbor Press, 2007. |
||
| + | *[[Evolution]] by Nicholas Barton, Derek Briggs, Jonathan Eisen, David Goldstein, and Nipam Patel. Cold Spring Harbor Press, 2007. |
||
| + | *[[Chromatin and Gene Regulation: Mechanisms in Epigenetics]] by Bryan Turner. Blackwell Publishing, 2002. |
||
| + | *[[Survival of the Sickest (book)|Survival of the Sickest]] by Dr. Sharon Moalem with Jonathan Prince, Published 2007 |
||
| + | *[http://www.horizonpress.com/epi Epigenetics] edited by J. Tost. Caister Academic Press, 2008. |
||
| + | * [http://www.cancermonthly.com/blog/2007/12/cancer-gene.html Gene and Epigene - The Next Cancer Therapy?] |
||
| + | *[http://www.horizonpress.com/rnareg RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity] edited by K. V. Morris. Caister Academic Press, 2008. |
||
| + | == Notes and references == |
||
| + | {{Reflist|2}} |
||
| + | ==External links== |
||
| − | [[image:Epigenome NoE tag1.gif|center]] |
||
| + | |||
| + | *[http://discovermagazine.com/2006/nov/cover DNA Is Not Destiny] - Discover Magazine cover story |
||
| + | *[http://www.bbc.co.uk/sn/tvradio/programmes/horizon/ghostgenes.shtml BBC - Horizon - 2005 - The Ghost In Your Genes] |
||
{{genarch}} |
{{genarch}} |
||
| − | |||
[[Category:Epigenetics]] |
[[Category:Epigenetics]] |
||
| + | <!-- |
||
[[de:Epigenetik]] |
[[de:Epigenetik]] |
||
[[es:Epigenética]] |
[[es:Epigenética]] |
||
[[fr:Épigénétique]] |
[[fr:Épigénétique]] |
||
| + | [[it:Epigenetica]] |
||
[[he:אפיגנטיקה]] |
[[he:אפיגנטיקה]] |
||
[[hu:Epigenetika]] |
[[hu:Epigenetika]] |
||
[[nl:Epigenetica]] |
[[nl:Epigenetica]] |
||
| + | [[ja:エピジェネティクス]] |
||
[[pl:Epigenetyka]] |
[[pl:Epigenetyka]] |
||
| − | [[ |
+ | [[pt:Epigenética]] |
| + | [[ur:بالاوراثیات]] |
||
| − | [[ja:エピジェネティクス]] |
||
| + | [[ru:Эпигенетика]] |
||
| + | [[sl:Epigenetika]] |
||
[[fi:Epigeneettinen periytyminen]] |
[[fi:Epigeneettinen periytyminen]] |
||
| + | [[sv:Epigenetik]] |
||
| + | [[zh:表觀遺傳學]] |
||
| + | --> |
||
{{enWP|Epigenics}} |
{{enWP|Epigenics}} |
||
Revision as of 07:07, 7 March 2008
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
- For the unfolding of an organism or the theory that plants and animals develop in this way, see Epigenesis (biology).
Epigenetics is a term in biology used to refer to features such as chromatin and DNA modifications that are stable over rounds of cell division but do not involve changes in the underlying DNA sequence of the organism.[1] These epigenetic changes play a role in the process of cellular differentiation, allowing cells to stably maintain different characteristics despite containing the same genomic material. Epigenetic features are inherited when cells divide despite a lack of change in the DNA sequence itself and, although most of these features are considered dynamic over the course of development in multicellular organisms, some epigenetic features show transgenerational inheritance and are inherited from one generation to the next.[2]
Specific epigenetic processes include paramutation, bookmarking, imprinting, gene silencing, X chromosome inactivation, position effect, reprogramming, transvection, maternal effects, the progress of carcinogenesis, many effects of teratogens, regulation of histone modifications and heterochromatin, and technical limitations affecting parthenogenesis and cloning.
Epigenetic research uses a wide range of molecular biologic techniques to further our understanding of epigenetic phenomena, including chromatin immunoprecipitation (together with its large-scale variants ChIP-on-chip and ChIP-seq), fluorescent in situ hybridization, methylation-sensitive restriction enzymes, DNA adenine methyltransferase identification (DamID) and bisulfite sequencing. Furthermore, the use of bioinformatic methods is playing an increasing role (computational epigenetics).
Etymology and definitions
The word "epigenetics" has been associated with many different definitions, and much of the confusion surrounding the use of the word "epigenetics" relates to the fact that it was originally defined to explain phenomena without knowing their molecular basis and with time became narrowly linked to certain phenomena as their molecular basis was discovered.[3]
The word "epigenetics" (as in "epigenetic landscape") was coined by C. H. Waddington in 1942 as a portmanteau of the words "genetics" and "epigenesis".[4] Epigenesis is an older word used to describe the differentiation of cells from a totipotent state in embryonic development (used in contrast to "preformationism"). At the time Waddington first used the term "epigenetics," the physical nature of genes and their role in heredity was not known. Epigenetics was Waddington's model of how genes within a multicellular organism interact with their surroundings to produce a phenotype. Because all cells within an organism inherit the same DNA sequences, cellular differentiation processes crucial for epigenesis rely strongly on epigenetic rather than genetic inheritance. Robin Holliday defined epigenetics as "the study of the mechanisms of temporal and spatial control of gene activity during the development of complex organisms."[5] Thus, the word "epigenetic" can be used to describe any aspect other than DNA sequence that influences the development of an organism.
Another usage of the word "epigenetics" was employed by the psychologist Erik Erikson, who developed an "epigenetic theory of human development," which focuses on psycho-social crises.
The modern usage of the word "epigenetic" is usually more narrow, referring to heritable traits (over rounds of cell division and sometimes transgenerationally) that do not involve changes to the underlying DNA sequence.[6] The Greek prefix "epi-" in the word "epigenetics" implies features that are "on top of" or "in addition to" genetics, and the current usage of the word reflects this—epigenetic traits exist on top of or in addition to the traditional molecular basis for inheritance.
The similarity of the word to "genetics" has generated many parallel usages. The "epigenome" is a parallel to the word "genome," and refers to the overall epigenetic state of a cell. The phrase "genetic code" has also been adapted—the "epigenetic code" has been used to describe the set of epigenetic features that create different phenotypes in different cells. Taken to its extreme, the "epigenetic code" could represent the total state of the cell, with the position of each molecule accounted for; more typically, the term is used in reference to systematic efforts to measure specific, relevant forms of epigenetic information such as the histone code or DNA methylation patterns.
Mechanisms
Several types of epigenetic inheritance systems may play a role in what has become known as cell memory:[7]
DNA methylation and chromatin remodeling
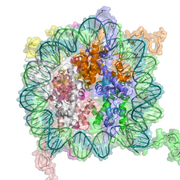
DNA associates with histone proteins to form chromatin.
Because the phenotype of a cell or individual is affected by which of its genes are transcribed, heritable transcription states can give rise to epigenetic effects. There are several layers of regulation of gene expression, one of which is remodeling of chromatin, the complex of DNA and the histone proteins with which it associates. Chromatin remodeling is initiated by one of two things:
- posttranslational modification of the amino acids that make up histone proteins,
- or the addition of methyl groups to the DNA, at CpG sites, to convert cytosine to 5-methylcytosine.
Whereas DNA is not completely stripped of nucleosomes during replication, it is possible that the remaining modified histones may act as templates, initiating identical modification of surrounding new histones after deposition. DNA methylation has a more clear method of propagation through the preferential methylation of hemimethylated symmetric sites by enzymes like Dnmt 1.
Although modifications occur throughout the histone sequence, the unstructured termini of histones (called histone tails) are particularly highly modified. These modifications include acetylation, methylation and ubiquitylation. Acetylation is the most highly studied of these modifications. For example, acetylation of the K14 and K9 lysines of the tail of histone H3 by histone acetyltransferase enzymes (HATs) is generally correlated with transcriptional competence.
One mode of thinking is that this tendency of acetylation to be associated with "active" transcription is biophysical in nature. Because lysine normally has a positive charge on the nitrogen at its end, lysine can bind the negatively charged phosphates of the DNA backbone and prevent them from repelling each other. The acetylation event converts the positively charged amine group on the side chain into a neutral amide linkage. This removes the positive charge causing the DNA to repel itself. When this occurs, complexes like SWI/SNF and other transcriptional factors can bind to the DNA, thus opening it up and exposing it to enzymes like RNA polymerase so transcription of the gene can occur.
In addition, the positively charged tails of histone proteins from one nucleosome may interact with the histone proteins on a neighboring nucleosome, causing them to pack closely. Lysine acetylation may interfere with these interactions, causing the chromatin structure to open up.
Lysine acetylation may also act as a beacon to recruit other activating chromatin modifying enzymes (and basal transcription machinery as well). Indeed, the bromodomain—a protein segment (domain) that specifically binds acetyl-lysine—is found in many enzymes that help activate transcription including the SWI/SNF complex (on the protein polybromo). It may be that acetylation acts in this and the previous way to aid in transcriptional activation.
The idea that modifications act as docking modules for related factors is borne out by histone methylation as well. Methylation of lysine 9 of histone H3 has long been associated with constitutively transcriptionally silent chromatin (constitutive heterochromatin). It has been determined that a chromodomain (a domain that specifically binds methyl-lysine) in the transcriptionally repressive protein HP1 recruits HP1 to K9 methylated regions. One example that seems to refute the biophysical model for acetylation is that tri-methylation of histone H3 at lysine 4 is strongly associated with (and required for full) transcriptional activation. Tri-methylation in this case would introduce a fixed positive charge on the tail.
It should be emphasized that differing histone modifications are likely to function in differing ways; acetylation at one position is likely to function differently than acetylation at another position. Also, multiple modifications may occur at the same time, and these modifications may work together to change the behavior of the nucleosome. The idea that multiple dynamic modifications regulate gene transcription in a systematic and reproducible way is called the histone code.
DNA methylation frequently occurs in repeated sequences, and may help to suppress 'junk DNA':[8] Because 5-methylcytosine is chemically very similar to thymidine, CpG sites are frequently mutated and become rare in the genome, except at CpG islands where they remain unmethylated. Epigenetic changes of this type thus have the potential to direct increased frequencies of permanent genetic mutation. DNA methylation patterns are known to be established and modified in response to environmental factors by a complex interplay of at least three independent DNA methyltransferases, DNMT1, DNMT3A and DNMT3B, the loss of any of which is lethal in mice.[9] DNMT1 is the most abundant methyltransferase in somatic cells,[10] localizes to replication foci,[11] has a 10–40-fold preference for hemimethylated DNA and interacts with the proliferating cell nuclear antigen (PCNA).[12] By preferentially modifying hemimethylated DNA, DNMT1 transfers patterns of methylation to a newly synthesized strand after DNA replication, and therefore is often referred to as the ‘maintenance' methyltransferase.[13] DNMT1 is essential for proper embryonic development, imprinting and X-inactivation.[9][14]
Chromosomal regions can adopt stable and heritable alternative states resulting in bistable gene expression without changes to the DNA sequence. Epigenetic control is often associated with alternative covalent modifications of histones. The stability and heritability of states of larger chromosomal regions are often thought to involve positive feedback where modified nucleosomes recruit enzymes that similarly modify nearby nucleosomes. A simplified stochastic model for this type of epigenetics is found here [15] .
Because DNA methylation and chromatin remodeling play such a central role in many types of epigenic inheritance, the word "epigenetics" is sometimes used as a synonym for these processes. However, this can be misleading. Chromatin remodeling is not always inherited, and not all epigenetic inheritance involves chromatin remodeling.[16]
It has been suggested that the histone code could be mediated by the effect of small RNAs. The recent discovery and characterization of a vast array of small (21- to 26-nt), non-coding RNAs suggests that there is an RNA component, possibly involved in epigenetic gene regulation. Small interfering RNAs can modulate transcriptional gene expression via epigenetic modulation of targeted promoters.[17]
RNA transcripts and their encoded proteins
Sometimes a gene, after being turned on, transcribes a product that (either directly or indirectly) maintains the activity of that gene. For example, Hnf4 and MyoD enhance the transcription of many liver- and muscle-specific genes, respectively, including their own, through the transcription factor activity of the proteins they encode. Other epigenetic changes are mediated by the production of different splice forms of RNA, or by formation of double-stranded RNA (RNAi). Descendants of the cell in which the gene was turned on will inherit this activity, even if the original stimulus for gene-activation is no longer present. These genes are most often turned on or off by signal transduction, although in some systems where syncytia or gap junctions are important, RNA may spread directly to other cells or nuclei by diffusion. A large amount of RNA and protein is contributed to the zygote by the mother during oogenesis or via nurse cells, resulting in maternal effect phenotypes. A smaller quantity of sperm RNA is transmitted from the father, but there is recent evidence that this epigenetic information can lead to visible changes in several generations of offspring.[18]
Prions
- For more details on this topic, see Prions.
Prions are infectious forms of proteins. Proteins generally fold into discrete units which perform distinct cellular functions, but some proteins are also capable of forming an infectious conformational state known as a prion. Although often viewed in the context of infectious disease, prions are more loosely defined by their ability to catalytically convert other native state versions of the same protein to an infectious conformational state. It is in this latter sense that they can be viewed as epigenetic agents capable of inducing a phenotypic change without a modification of the genome.[19]
Fungal prions are considered epigenetic because the infectious phenotype caused by the prion can be inherited without modification of the genome. PSI+ and URE3, discovered in yeast in 1965 and 1971, are the two best studied of this type of prion.[20][21] Prions can have a phenotypic effect through the sequestration of protein in aggregates, thereby reducing that protein's activity. In PSI+ cells, the loss of the Sup35 protein (which is involved in termination of translation) causes ribosomes to have a higher rate of read-through of stop codons, an effect which results in suppression of nonsense mutations in other genes.[22] The ability of Sup35 to form prions may be a conserved trait. It could confer an adaptive advantage by giving cells the ability to switch into a PSI+ state and express dormant genetic features normally terminated by premature stop codon mutations.[23][24]
Structural inheritance systems
- For more details on this topic, see Structural inheritance.
In ciliates such as Tetrahymena and Paramecium, genetically identical cells show heritable differences in the patterns of ciliary rows on their cell surface. Experimentally altered patterns can be transmitted to daughter cells. It seems existing structures act as templates for new structures. The mechanisms of such inheritance are unclear, but reasons exist to assume that multicellular organisms also use existing cell structures to assemble new ones.[25]
Functions and consequences
Development
Somatic epigenetic inheritance, particularly through DNA methylation and chromatin remodeling, is very important in the development of multicellular eukaryotic organisms. The genome sequence is static (with some notable exceptions), but cells differentiate in many different types, which perform different functions, and respond differently to the environment and intercellular signalling. Thus, as individuals develop, morphogens activate or silence genes in an epigenetically heritable fashion, giving cells a "memory". In mammals, most cells terminally differentiate, with only stem cells retaining the ability to differentiate into several cell types ("totipotency" and "multipotency"). In mammals, some stem cells continue producing new differentiated cells throughout life, but mammals are not able to respond to loss of some tissues, for example, the inability to regenerate limbs, which some other animals are capable of. Unlike animals, plant cells do not terminally differentiate, remaining totipotent with the ability to give rise to a new individual plant. While plants do utilise many of the same epigenetic mechanisms as animals, such as chromatin remodeling, it has been hypothesised that plant cells do not have "memories", resetting their gene expression patterns at each cell division using positional information from the environment and surrounding cells to determine their fate.[26]
Medicine
Epigenetics has many and varied potential medical applications. Congenital genetic disease is well understood, and it is also clear that epigenetics can play a role, for example, in the case of Angelman syndrome and Prader-Willi syndrome. These are normal genetic diseases caused by gene deletions, but are unusually common because individuals are essentially hemizygous because of genomic imprinting, and therefore a single gene knock out is sufficient to cause the disease, where most cases would require both copies to be knocked out.[27]
Evolution
Although epigenetics in multicellular organisms is generally thought to be a mechanism involved in differentiation, with epigenetic patterns "reset" when organisms reproduce, there have been some observations of transgenerational epigenetic inheritance (e.g., the phenomenon of paramutation observed in maize). Although most of these multigenerational epigenetic traits are gradually lost over several generations, the possibility remains that multigenerational epigenetics could be another aspect to evolution and adaptation. These effects may require enhancements to the standard conceptual framework of the modern evolutionary synthesis.[28][29]
Epigenetic features may play a role in short-term adaptation of species by allowing for reversible phenotype variability. The modification of epigenetic features associated with a region of DNA allows organisms, on a multigenerational time scale, to switch between phenotypes that express and repress that particular gene.[30] Whereas the DNA sequence of the region is not mutated, this change is reversible. It has also been speculated that organisms may take advantage of differential mutation rates associated with epigenetic features to control the mutation rates of particular genes.[30]
Epigenetic changes have also been observed to occur in response to environmental exposure—for example, mice given some dietary supplements have epigenetic changes affecting expression of the agouti gene, which affects their fur color, weight, and propensity to develop cancer.[31][32] Although there is no reason to suspect that such a process is anything other than a product of genetics, and therefore orthodox evolutionary mechanisms, the observation of trans-generational epigenetic change occurring in response to environmental factors is reminiscent of the 19th century Lamarckian hypothesis of inheritance and evolution.
Epigenetic effects in humans
Some human disorders are associated with genomic imprinting, a phenomenon in mammals where the father and mother contribute different epigenetic patterns for specific genomic loci in their germ cells.[33] The most well-known case of imprinting in human disorders is that of Angelman syndrome and Prader-Willi syndrome—both can be produced by the same genetic mutation, chromosome 15q partial deletion, and the particular syndrome that will develop depends on whether the mutation is inherited from the child's mother or from their father.[34] This is due to the presence of genomic imprinting in the region. Beckwith-Wiedemann syndrome is also associated with genomic imprinting, often caused by abnormalities in maternal genomic imprinting of a region on chromosome 11.
Transgenerational epigenetic observations
Marcus Pembrey and colleagues also observed that the paternal (but not maternal) grandsons of Swedish boys who were exposed to famine in the 19th century were less likely to die of cardiovascular disease; if food was plentiful then diabetes mortality in the grandchildren increased, suggesting that this was a transgenerational epigenetic inheritance.[35]
Cancer and developmental abnormalities
A variety of compounds are considered as epigenetic carcinogens—they result in an increased incidence of tumors, but they do not show mutagen activity (toxic compounds or pathogens that cause tumors incident to increased regeneration should also be excluded). Examples include diethylstilbestrol, arsenite, hexachlorobenzene, and nickel compounds.
Many teratogens exert specific effects on the fetus by epigenetic mechanisms.[36][37] While epigenetic effects may preserve the effect of a teratogen such as diethylstilbestrol throughout the life of an affected child, the possibility of birth defects resulting from exposure of fathers or in second and succeeding generations of offspring has generally been rejected on theoretical grounds and for lack of evidence.[38] However, a range of male-mediated abnormalities have been demonstrated, and more are likely to exist.[39] FDA label information for Vidaza(tm), a formulation of 5-azacitidine (an unmethylatable analog of cytidine that causes hypomethylation when incorporated into DNA) states that "men should be advised not to father a child" while using the drug, citing evidence in treated male mice of reduced fertility, increased embryo loss, and abnormal embryo development. In rats, endocrine differences were observed in offspring of males exposed to morphine.[40] In mice, second generation effects of diethylstilbesterol have been described occurring by epigenetic mechanisms.[41]
Epigenetics in microorganisms
Bacteria make widespread use of postreplicative DNA methylation for the epigenetic control of DNA-protein interactions. Bacteria make use of DNA adenine methylation (rather than DNA cytosine methylation) as an epigenetic signal. DNA adenine methylation is important in bacteria virulence in organisms such as Escherichia coli, Salmonella, Vibrio, Yersinia, Haemophilus, and Brucella. In Alphaproteobacteria, methylation of adenine regulates the cell cycle and couples gene transcription to DNA replication. In Gammaproteobacteria, adenine methylation provides signals for DNA replication, chromosome segregation, mismatch repair, packaging of bacteriophage, transposase activity and regulation of gene expression.[42] [43]
The yeast prion PSI is generated by a conformational change of a translation termination factor, which is then inherited by daughter cells. This can provide a survival advantage under adverse conditions. This is an example of epigenetic regulation enabling unicellular organisms to respond rapidly to environmental stress. Prions can be viewed as epigenetic agents capable of inducing a phenotypic change without modification of the genome.[43]
See also
- Histone code
- Baldwinian evolution
- Barbara McClintock
- Centromere
- Evolutionary developmental psychology
- Molecular biology
- Somatic epitype
- Weismann barrier
Further reading
- Oskar Hertwig, 1849-1922. Biological problem of today: preformation or epigenesis? The basis of a theory of organic development. W. Heinemann: London, 1896.
- R. Jaenisch and A. Bird (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33 (Suppl) 245-254.
- Joshua Lederberg, "The Meaning of Epigenetics", The Scientist 15(18):6, Sep. 17, 2001.
- R. J. Sims III, K. Nishioka and D. Reinberg (2003) Histone lysine methylation: a signature for chromatin function. Trends Genet. 19, 629-637.
- B. D. Strahl and C. D. Allis (2000) The language of covalent histone modifications. Nature 403, 41-45.
- C.H. Waddington (1942), "The epigenotype". Endeavour 1, 18–20.
- B. McClintock (1978) Mechanisms that Rapidly Reorganize the Genome. Stadler Symposium vol 10:25-48
- G.W. Grimes; K.J. Aufderheide; Cellular Aspects of Pattern Formation: the Problem of Assembly. Monographs in Developmental Biology, Vol. 22. Karger, Basel (1991)
- Eva Jablonka and Marion J. Lamb Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life The MIT Press (2005) ISBN 978-0262101073
- Article on The Philosophy of Molecular and Developmental Biology to appear in Blackwell’s Guide to Philosophy of Science,. P.K. Machamer and M. Silberstein (Eds).
- Epigenetics edited by C. David Allis, Thomas Jenuwein, Danny Reinberg, and Marie-Laure Caparros. Cold Spring Harbor Press, 2007.
- Evolution by Nicholas Barton, Derek Briggs, Jonathan Eisen, David Goldstein, and Nipam Patel. Cold Spring Harbor Press, 2007.
- Chromatin and Gene Regulation: Mechanisms in Epigenetics by Bryan Turner. Blackwell Publishing, 2002.
- Survival of the Sickest by Dr. Sharon Moalem with Jonathan Prince, Published 2007
- Epigenetics edited by J. Tost. Caister Academic Press, 2008.
- Gene and Epigene - The Next Cancer Therapy?
- RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity edited by K. V. Morris. Caister Academic Press, 2008.
Notes and references
- ↑ Adrian Bird (2007). Perceptions of epigenetics. Nature 447: 396-398. PMID 17522671
- ↑ V.L. Chandler (2007). Paramutation: From Maize to Mice. Cell 128: 641-645.
- ↑ Roloff, T.C., Nuber, U.A., 2005 Chromatin , epigenetics and stem cells. Eur J Cell Biol. 84, 123-135
- ↑ C.H. Waddington (1942). The epigenotype. Endeavour 1: 18-20.
- ↑ Holliday, R., 1990. Mechanisms for the control of gene activity during development. Biol. Rev. Cambr. Philos. Soc. 65, 431-471
- ↑ Russo, V.E.A., Martienssen, R.A., Riggs, A.D., 1996 Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory Press, Plainview, NY.
- ↑ Jablonka, E, Lamb MJ and Lachmann M (September 1992). Evidence, mechanisms and models for the inheritance of acquired characteristics. J. Theoret. Biol. 158 (2): 245–268.
- ↑ Chédin, F (1992). The Chedin Laboratory. URL accessed on 2006-12-28.
- ↑ 9.0 9.1 Li, E, Bestor TH and Jaenisch R (June 1992). Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69 (6): 915–926.
- ↑ Robertson, KD, Uzyolgi E, Lian G et al (June 1999). The human DNA methyltransferases (DNMTs) 1, 3a, 3b: Coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res 27 (11): 2291–2298.
- ↑ Leonhardt, H, Page AW, Weier HU, Bestor TH (November 1992). A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 71 (5): 865–873.
- ↑ Chuang, LS, Ian HI, Koh TW et al (September 1997). Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1. Science 277 (5334): 1996–2000.
- ↑ Robertson, KD, Wolffe AP (October 2000). DNA methylation in health and disease. Nat Rev Genet 1 (1): 11–19.
- ↑ Li, E, Beard C and Jaenisch R (December 1993). Role for DNA methylation in genomic imprinting. Nature 366 (6453): 362–365.
- ↑ I.B. Dodd, M.A. Micheelsen, K. Sneppen and G. Thon (2007). Theoretical Analysis of Epigenetic Cell Memory by Nucleosome Modification Cell 129:813-822.
- ↑ Mark Ptashne, 2007. On the use of the word ‘epigenetic’. Current Biology, 17(7):R233-R236. DOI:10.1016/j.cub.2007.02.030
- ↑ Morris KV (2008). "Epigenetic Regulation of Gene Expression" RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity, Caister Academic Press. ISBN 978-1-904455-25-7.
- ↑ Choi CQ. The Scientist: RNA can be hereditary molecule. The Scientist. URL accessed on 2006.
- ↑ A. Yool and W.J. Edmunds (1998). Epigenetic inheritance and prions. Journal of Evolutionary Biology 11: 241-242.
- ↑ B.S. Cox (1965). [PSI], a cytoplasmic suppressor of super-suppression in yeast. Heredity 20: 505-521.
- ↑ F. Lacroute (1971). Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. Journal of Bacteriology 106: 519-522.
- ↑ S.W. Liebman and F. Sherman (1979). Extrachromosomal psi+ determinant suppresses nonsense mutations in yeast. Journal of Bacteriology 139 (3): 1068-1071. Free full text available
- ↑ H.L. True and S.L. Lindquist (2000). A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407: 477-483.
- ↑ J. Shorter and S. Lindquist (2005). Prions as adaptive conduits of memory and inheritance. Nature Reviews Genetics 6 (6): 435-450.
- ↑ Oyama, Susan; Paul E. Griffiths, Russell D. Gray (2001). Cycles of Contingency: Developmental Systems and Evolution, MIT Press.
- ↑ Silvia Costa and Peter Shaw. 2006. 'Open Minded' cells: how cells can change fate. Trends in Cell Biology 17(3):101-106. DOI:10.1016/j.tcb.2006.12.005
- ↑ OMIM 105830
- ↑ Jablonka, Eva; Marion J. Lamb (2005). Evolution in Four Dimensions, MIT Press. ISBN 0-262-10107-6.
- ↑ See also Denis Noble The Music of Life see esp pp93-8 and p48 where he cites Jablonka & Lamb and Massimo Pigliucci's review of Jablonka and Lamb in Nature 435, 565-566 (2 June 2005)
- ↑ 30.0 30.1 O.J. Rando and K.J. Verstrepen (2007). Timescales of Genetic and Epigenetic Inheritance. Cell 128: 655-668.
- ↑ Cooney, CA, Dave, AA, and Wolff, GL (2002). Maternal Methyl Supplements in Mice Affect Epigenetic Variation and DNA Methylation of Offspring. Journal of Nutrition 132: 2393S-2400S.available online
- ↑ Waterland RA and Jirtle RL (August 2003). Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Molecular and Cellular Biology 23 (15): 5293-5300.
- ↑ A.J. Wood and A.J. Oakey (2006). Genomic imprinting in mammals: Emerging themes and established theories. PLOS Genetics 2 (11): 1677-1685. available online
- ↑ J.H.M. Knoll, R.D. Nicholls, R.E. Magenis, J.M. Graham Jr, M. Lalande, S.A. Latt (1989). Angelman and Prader-Willi syndromes share a common chromosome deletion but differ in parental origin of the deletion. American Journal of Medical Genetics 32: 285-290.
- ↑ Pembrey ME, Bygren LO, Kaati G, et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 2006; 14: 159-66. PMID 16391557. Robert Winston refers to this study in a lecture; see also discussion at Leeds University, here
- ↑ Bishop, JB, Witt KL and Sloane RA (December 1997). Genetic toxiticities of human teratogens. Mutat Res 396 (1-2): 9–43.
- ↑ Gurvich, N, Berman MG, Wittner BS et al (July 2004). Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J 19 (9): 1166–1168.
- ↑ Smithells, D (November 1998). Does thalidomide cause second generation birth defects?. Drug Saf 19 (5): 339–341.
- ↑ Friedler, G (December 1996). Paternal exposures: impact on reproductive and developmental outcome. An overview.. Pharmacol Biochem Behav 55 (4): 691–700.
- ↑ Cicero, TJ, Adams NL, Giodarno A et al (March 1991). Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring. J Pharmacol Exp Ther. 256 (3): 1086–1093.
- ↑ Newbold, RR, Padilla-Banks E and Jefferson WN (June 2006). Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 147 (6 Suppl): S11–S17.
- ↑ Casadesus J and Low D (September 2006). Epigenetic Gene Regulation in the Bacterial World. Microbiol Mol Biol Rev 70 (3): 830-856.
- ↑ 43.0 43.1 Tost J (editor). (2008). Epigenetics, Caister Academic Press. ISBN 978-1-904455-23-3 .
External links
- DNA Is Not Destiny - Discover Magazine cover story
- BBC - Horizon - 2005 - The Ghost In Your Genes
The development of phenotype
|
|---|
| Key concepts: Genotype-phenotype distinction | Norms of reaction | Gene-environment interaction | Heritability | Quantitative genetics |
| Genetic architecture: Dominance relationship | Epistasis | Polygenic inheritance | Pleiotropy | Plasticity | Canalisation | Fitness landscape |
| Non-genetic influences: Epigenetic inheritance | Epigenetics | Maternal effect | dual inheritance theory |
| Developmental architecture: Segmentation | Modularity |
| Evolution of genetic systems: Evolvability | Mutational robustness | Evolution of sex |
| Influential figures: C. H. Waddington | Richard Lewontin |
| Debates: Nature versus nurture |
| List of evolutionary biology topics |
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |