(refs) |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" |
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" |
||
| − | ! {{chembox header}} | |
+ | ! {{chembox header}} | Dopamine |
|- |
|- |
||
| − | | align="center" colspan="2" bgcolor="#ffffff" | [[Image: |
+ | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:Dopamine2.svg|200px|Dopamine]] <br>[[Image:Dopamine-3d-CPK.png|175px]] |
|- |
|- |
||
! {{chembox header}} | General |
! {{chembox header}} | General |
||
| Line 12: | Line 12: | ||
|- |
|- |
||
| Other names |
| Other names |
||
| − | | 2-(3,4-dihydroxyphenyl)ethylamine;<br/> 3,4-dihydroxyphenethylamine;<br/> 3-hydroxytyramine; DA; Intropin<br/> Revivan |
+ | | 2-(3,4-dihydroxyphenyl)ethylamine;<br/> 3,4-dihydroxyphenethylamine;<br/> 3-hydroxytyramine; DA; Intropin<br/> Revivan; Oxytyramine |
|- |
|- |
||
| [[Chemical formula|Molecular formula]] |
| [[Chemical formula|Molecular formula]] |
||
| Line 18: | Line 18: | ||
|- |
|- |
||
| [[Simplified molecular input line entry specification|SMILES]] <!-- mostly for organic compounds, omit otherwise --> |
| [[Simplified molecular input line entry specification|SMILES]] <!-- mostly for organic compounds, omit otherwise --> |
||
| + | | NCCc1ccc(O)c(O)c1 |
||
| − | | C1=CC(=C(C=C1CCN)O)O |
||
|- |
|- |
||
| [[Molar mass]] |
| [[Molar mass]] |
||
| − | | 153. |
+ | | 153.178 g/mol |
|- |
|- |
||
| Appearance |
| Appearance |
||
| Line 32: | Line 32: | ||
|- |
|- |
||
| [[Density]] and [[Phase (matter)|phase]] |
| [[Density]] and [[Phase (matter)|phase]] |
||
| − | | ? g/cm |
+ | | ? g/cm³, ? <!-- ? g/cm³, solid / ? g/ml, liquid / ? g/l, gas --> |
|- |
|- |
||
| [[Soluble|Solubility]] in [[Water (molecule)|water]] |
| [[Soluble|Solubility]] in [[Water (molecule)|water]] |
||
| − | | |
+ | | 60.0 g/100 ml (? °C), solid <!-- at least put miscible with, not soluble in --> |
|- |
|- |
||
<!-- | Other solvents e.g. [[ethanol]], [[acetone]] --> |
<!-- | Other solvents e.g. [[ethanol]], [[acetone]] --> |
||
| Line 75: | Line 75: | ||
|- |
|- |
||
| [[Material safety data sheet|MSDS]] |
| [[Material safety data sheet|MSDS]] |
||
| − | | [[ |
+ | | [[Material Safety Data Sheet|External MSDS]] <!-- please replace with proper link--> |
|- |
|- |
||
| Main [[Worker safety and health|hazard]]s |
| Main [[Worker safety and health|hazard]]s |
||
| Line 92: | Line 92: | ||
| UX1088000 |
| UX1088000 |
||
|- |
|- |
||
| − | ! {{chembox header}} | |
+ | ! {{chembox header}} | Supplementary data page |
|- |
|- |
||
| − | | |
+ | | Structure and<br/>properties |
| [[Refractive index|''n'']], [[Dielectric constant|ε<sub>r</sub>]], etc. |
| [[Refractive index|''n'']], [[Dielectric constant|ε<sub>r</sub>]], etc. |
||
|- |
|- |
||
| − | | [[ |
+ | | [[Thermodynamic properties|Thermodynamic<br/>data]] |
| Phase behaviour<br>Solid, liquid, gas |
| Phase behaviour<br>Solid, liquid, gas |
||
|- |
|- |
||
| − | | |
+ | | Spectral data |
| [[UV/VIS spectroscopy|UV]], [[Infrared spectroscopy|IR]], [[NMR spectroscopy|NMR]], [[Mass spectrometry|MS]] |
| [[UV/VIS spectroscopy|UV]], [[Infrared spectroscopy|IR]], [[NMR spectroscopy|NMR]], [[Mass spectrometry|MS]] |
||
|- |
|- |
||
| Line 111: | Line 111: | ||
| ? <!-- Put in other oxidn states of same element e.g. [[iron(III) chloride]], also for related metals such as [[manganese(II) chloride]], [[cobalt(II) chloride]], ruthenium(III) chloride--> |
| ? <!-- Put in other oxidn states of same element e.g. [[iron(III) chloride]], also for related metals such as [[manganese(II) chloride]], [[cobalt(II) chloride]], ruthenium(III) chloride--> |
||
|- |
|- |
||
| + | | Related |
||
| − | | Related [[?]] <!-- PLEASE INSERT FUNCTIONAL GROUP (e.g. [[aldehyde]]) FOR ORGANICS, please omit if not applicable --> |
||
| + | | [[Tyramine]],<br/>[[octopamine]],<br/>[[norepinephrine]] (noradrenaline) |
||
| − | | ? <!-- Insert related organics e.g. on formaldehyde page put [[acetaldehyde]] --> |
||
|- |
|- |
||
| {{chembox header}} | <small>Except where noted otherwise, data are given for<br> materials in their [[standard state|standard state (at 25 °C, 100 kPa)]]<br/>[[wikipedia:Chemical infobox|Infobox disclaimer and references]]</small> |
| {{chembox header}} | <small>Except where noted otherwise, data are given for<br> materials in their [[standard state|standard state (at 25 °C, 100 kPa)]]<br/>[[wikipedia:Chemical infobox|Infobox disclaimer and references]]</small> |
||
| Line 118: | Line 118: | ||
|} |
|} |
||
| + | '''Dopamine''' is a [[hormone]] and [[neurotransmitter]] occurring in a wide variety of animals, including both vertebrates and invertebrates. In chemical structure, it is a [[phenethylamine]]. |
||
| ⚫ | |||
| ⚫ | In the [[human brain|brain]], dopamine functions as a [[neurotransmitter]], activating the five types of [[dopamine receptor]]s — D1, D2, D3, D4 and D5, and their variants. Dopamine is produced in several areas of the brain, including the [[substantia nigra]]. Dopamine is also a [[neurohormone]] released by the [[hypothalamus]]. Its main function as a hormone is to inhibit the release of [[prolactin]] from the anterior lobe of the [[pituitary]]. |
||
| ⚫ | Dopamine can be supplied as a [[medication]] that acts on the [[sympathetic nervous system|sympathetic]] [[nervous system]], producing effects such as increased [[heart]] rate and [[blood pressure]]. However, |
||
| ⚫ | Dopamine can be supplied as a [[medication]] that acts on the [[sympathetic nervous system|sympathetic]] [[nervous system]], producing effects such as increased [[heart]] rate and [[blood pressure]]. However, because dopamine cannot cross the [[blood-brain barrier]], dopamine given as a drug does not directly affect the [[central nervous system]]. To increase the amount of dopamine in the brains of patients with diseases such as [[Parkinson's disease]] and Dopa-Responsive [[dystonia]], L-DOPA ([[levodopa]]), which is the precursor of dopamine, can be given because it can cross the [[blood-brain barrier]]. |
||
| ⚫ | |||
| ⚫ | |||
| + | == History == |
||
| − | As a member of the [[catecholamine]] family, dopamine is a precursor to [[epinephrine]] ([[adrenaline]]) and [[norepinephrine]] ([[noradrenaline]]) in the biosynthetic pathways for these neurotransmitters. [[Arvid Carlsson]] won a share of the [[2000]] [[Nobel Prize in Physiology or Medicine]] for showing that dopamine is not just a precursor to these, but a neurotransmitter as well. |
||
| + | Dopamine was discovered by [[Arvid Carlsson]] and Nils-Åke Hillarp at the Laboratory for Chemical Pharmacology of the National Heart Institute of Sweden, in 1952. It was named Dopamine because it was a [[monoamine]], and its synthetic precursor was 3,4-dihydroxyphenylalanine ([[L-DOPA]]).<ref> Benes, F.M. Carlsson and the discovery of dopamine.'' Trends in Pharmacological Sciences'', Volume 22, Issue 1, 1 January 2001, Pages 46-47.</ref> [[Arvid Carlsson]] was awarded the [[2000]] [[Nobel Prize in Physiology or Medicine]] for showing that dopamine is not just a precursor of [[norepinephrine]] (noradrenaline) and [[epinephrine]] (adrenaline) but a neurotransmitter, as well. |
||
| − | Dopamine is synthesized in the body (mainly by nervous tissue and adrenal glands) first by the dehydration of the amino acid [[tyrosine]] to DOPA by [[tyrosine hydroxylase]] and then by the [[decarboxylation]] of [[DOPA]] by [[aromatic-L-amino-acid decarboxylase]]. In [[neuron]]s, dopamine is packaged after synthesis into vesicles, which are then released in response to the presynaptic [[action potential]]. The inactivation mechanism of neurotransmission are 1) uptake via a specific transporter; 2) enzymatic breakdown; and 3) diffusion. Uptake back to the presynaptic neuron via the [[dopamine transporter]] is the major role in the inactivation of dopamine neurotransmission. The recycled dopamine will face either breakdown by an enzyme or be re-packaged into vesicles and reused. |
||
| + | Dopamine was first synthesized artificially in 1910 by George Barger and James Ewens at Wellcome Laboratories in London, England.<ref>[http://movementdisorders.org/education/onlinecme/levodopa/print.pdf Fahn, Stanley, "The History of Levodopa as it Pertains to Parkinson’s Disease," Movement Disorder Society’s 10th International Congress of Parkinson's Disease and Movement Disorders on November 1, 2006, in Kyoto, Japan.]</ref> |
||
| ⚫ | |||
| + | [[Image:Catecholamines biosynthesis.svg|thumb|right|400px|Biosynthesis of Dopamine]] |
||
| − | ==Dopamine pathways in the brain== |
||
| − | Dopamine pathways originate from groups of cells in the [[rostral]] areas of the brain. These groups were given the titles [[A8]], [[A9]], and [[A10]] by the Swedish neuoanatomists Falck and Hillarp who developed techniques forvisualizing catecholamine cells in the brain in the 1960s. They showed that Dopamine neurones are organised into pathways in the brain. |
||
| + | === Name and family === |
||
| ⚫ | |||
| ⚫ | |||
| + | As a member of the [[catecholamine]] family, dopamine is a precursor to [[norepinephrine]] (noradrenaline) and then [[epinephrine]] (adrenaline) in the biosynthetic pathways for these neurotransmitters. |
||
| + | === Biosynthesis === |
||
| − | ==Functions of dopamine in the brain== |
||
| − | Relatively few brain cell contain dopamine, of over 10 billion cells in the human brain, only 1 million contain it. Nevertheless, they play an important role in our behaviour. |
||
| + | Dopamine is biosynthesized in the body (mainly by nervous tissue and the [[adrenal medulla|medulla]] of the [[adrenal gland]]s) first by the hydration of the amino acid [[L-tyrosine]] to L-DOPA via the enzyme tyrosine 3-monooxygenase, also known as [[tyrosine hydroxylase]], and then by the [[decarboxylation]] of [[L-DOPA]] by [[aromatic L-amino acid decarboxylase]] (which is often referred to as dopa decarboxylase). In some neurons, dopamine is further processed into [[norepinephrine]] by [[dopamine beta-hydroxylase]]. |
||
| + | In [[neuron]]s, dopamine is packaged after synthesis into [[Vesicle (biology)|vesicles]], which are then released into the [[synapse]] in response to a presynaptic [[action potential]]. |
||
| − | ===Role in movement=== |
||
| − | Dopamine is critical to the way the brain controls our movements and is a crucial part of the [[basal ganglia]] motor loop. Shortage of dopamine, particularly the death of dopamine neurons in the [[nigrostriatal pathway]], causes [[Parkinson's disease]], in which a person loses the ability to execute smooth, controlled movements. |
||
| − | === |
+ | === Inactivation and degradation === |
| ⚫ | In the [[frontal lobe |
||
| + | [[Image:Dopamine degradation.svg|thumb|right|500px]] |
||
| ⚫ | |||
| ⚫ | Dopamine is the primary neuroendocrine regulator of the secretion of [[prolactin]] from the [[anterior pituitary]] gland. Dopamine produced by neurons in the [[arcuate nucleus]] of the |
||
| + | Dopamine is inactivated by reuptake via the [[dopamine transporter]], then enzymatic breakdown by [[catechol-O-methyl transferase]] (COMT) and [[monoamine oxidase]] (MAO). Dopamine that is not broken down by enzymes is repackaged into vesicles for reuse. |
||
| − | ===Role in pleasure and motivation=== |
||
| ⚫ | Dopamine is commonly associated with the ''pleasure system'' of the brain, providing feelings of enjoyment and [[reinforcement]] to motivate |
||
| + | Dopamine may also simply [[diffuse]] away from the [[synapse]], and help to regulate blood pressure. |
||
| − | However, the idea that dopamine is the 'reward chemical' of the brain, a view held by many during early stages of its research, now seems too simple. Dopamine is released when unpleasant or aversive stimuli are encountered, so it is not associated only with 'rewards' or pleasure. Recent research has begun to examine whether or not the firing of dopamine neurons might function as a reward-prediction error signal, based on evidence that, when a reward is greater than expected, there is an increase in the firing of certain dopamine neurons (in contrast to when there is a lesser-than-expected reward, and there is a marked decrease in the firing of the same neurons). Some argue that dopamine may be involved in [[desire]] rather than [[pleasure]]. |
||
| + | ==Functions in the brain== <!-- Neurotransmitter systems has a link to here --> |
||
| − | Confusion of dopamine's role in pleasure comes from studies performed on animals. It has been shown experimentally that when the dopaminergic system of a rat is selectively abolished it will stop eating. However when the rat is force fed food it will still display the proper facial expressions which indicate whether they like or dislike it. Conversely mutant hyperdopaminergic mice show higher wanting of food but not liking.{{Fn|1}} This research was taken to mean that dopamine mediated desire and incentive salience instead of pleasure. In humans, though, drugs that reduce dopamine activity (e.g., [[antipsychotic]]s) have been shown to induce both amotivation (lack of desire) as well as [[anhedonia]] (inability to experience pleasure).{{Fn|2}} The selective D2/D3 agonists pramipexole and ropinirole have anti-anhedonic and pro-motivational properties as measured by the Snaith-Hamilton Pleasure Scale.{{Fn|3}} Opioid and cannabinoid transmission instead of dopamine is believed to be what modulates food reward and palatability (liking).{{Fn|4}} This explains why animals would still have the same liking of food independent of brain dopamine concentrations. Other pleasures are likely dependent on dopamine. Libido can be increased by drugs that enhance dopaminergic functioning but not by ones that affect opioid peptides or other neurotransmitters. Sociability is also closely tied to dopamine neurotransmission. Low D2 receptor binding is found in people with social anxiety. Traits common to negative schizophrenia (social withdrawal, apathy, anhedonia) are thought to be related to a hypodopaminergic state in certain areas of the brain. In instances of bipolar hypomania subjects can become hypersocial as well as hypersexual. This is also believed to be due to an increase in dopamine, because it can be alleviated with dopamine blocking antipsychotics. |
||
| + | Dopamine has many functions in the brain, including important roles in behavior and [[cognition]], [[motor activity]], [[motivation]] and [[reward system|reward]], regulation of [[milk]] production, [[sleep]], [[Mood (psychology)|mood]], [[attention]], and [[learning]]. Dopaminergic neurons (i.e., neurons whose primary neurotransmitter is dopamine) are present chiefly in the [[ventral tegmental area]] (VTA) of the [[midbrain]], [[substantia nigra|substantia nigra pars compacta]], and [[arcuate nucleus]] of the hypothalamus. |
||
| + | The phasic responses of dopamine neurons are observed when an unexpected reward is presented. These responses transfer to the onset of a [[Classical conditioning|conditioned stimulus]] after repeated pairings with the reward. Further, dopamine neurons are depressed when the expected reward is omitted. Thus, dopamine neurons seem to [[encoding|encode]] the prediction error of rewarding outcomes. In nature, we learn to repeat behaviors that lead to maximize rewards. Dopamine is therefore believed to provide a teaching signal to parts of the brain responsible for acquiring new behavior. [[Temporal difference learning]] provides a computational model describing how the prediction error of dopamine neurons is used as a teaching signal. |
||
| − | Other theories suggest that the crucial role of dopamine may be in predicting pleasurable activity. Related theories argue that dopamine function may be involved in the salience ('noticeableness') of perceived objects and events, with potentially important stimuli (including rewarding things, but also things that may be dangerous or a threat) appearing more noticeable or more important. This theory argues that dopamine assists decision-making by influencing the priority of such stimuli to the person concerned. |
||
| + | In insects, a similar reward system exists, using [[octopamine]], a [[Quantitative structure-activity relationship|chemical relative]] of dopamine.<ref name="octopamine-honeybee">{{cite journal |author=Barron AB, Maleszka R, Vander Meer RK, Robinson GE |title=Octopamine modulates honey bee dance behavior |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=104 |issue=5 |pages=1703-7 |year=2007 |pmid=17237217 |doi=10.1073/pnas.0610506104}}</ref> |
||
| − | However, these theories are based on correlational, rather than causal, experimental evidence. The available evidence that examined causal relationships between dopamine and motivation does not seem to agree with any of the above-stated theories. For example, pharmacological blockade of brain dopamine receptors increases rather than decreases drug-taking behavior. The theories viewing dopamine as the mediator of 'desire/wanting,' 'predicting pleasurable activity,' 'noticeableness' or 'decision-making' cannot adequately explain this experimental evidence. Thus, the functional role of dopamine in motivation remains controversial. |
||
| + | === Anatomy === |
||
| − | Deficits in dopamine levels have also been implicated as one of several possible causes for [[Attention deficit disorder]], and some types of medications used to treat ADD and ADHD will help to stimulate dopaminergic systems, leading to potentially heightened, but preferably not distorted, sensation, for those who may be afflicted by it and be receiving treatment for it. |
||
| + | Dopaminergic neurons form a [[neurotransmitter system]] which originates in [[substantia nigra pars compacta]], [[ventral tegmental area]] (VTA) and hypothalamus. These project [[axon]]s to large areas of the brain through some major pathways: |
||
| ⚫ | |||
| − | Disruption to the dopamine system has also been strongly linked to [[psychosis]] and [[schizophrenia]]. Dopamine neurons in the [[mesolimbic pathway]] are particularly associated with these conditions. This is partly due to the discovery of a class of drugs called the [[phenothiazine]]s (which block D<sub>2</sub> [[dopamine receptor]]s) that can reduce psychotic symptoms, and partly due to the finding that drugs such as [[amfetamine|amphetamine]] and [[cocaine]] (which are known to greatly increase dopamine levels) can cause psychosis. Because of this, most modern [[antipsychotic]] medication is designed to block dopamine function to varying degrees. Blocking the D<sub>2</sub> [[dopamine receptor]] is known to cause relapse in patients that have achieved remission from depression, and such blocking also counteracts the effectiveness of [[Selective serotonin reuptake inhibitor|SSRI]] medication. |
||
| − | See the article on the [[dopamine hypothesis of psychosis]] for a wider discussion of this topic. |
||
| − | |||
| ⚫ | |||
| ⚫ | |||
| − | |||
| ⚫ | |||
| − | |||
| − | ==Major dopamine pathways== |
||
* [[Mesocortical pathway]] |
* [[Mesocortical pathway]] |
||
* [[Mesolimbic pathway]] |
* [[Mesolimbic pathway]] |
||
| Line 180: | Line 170: | ||
* [[Tuberoinfundibular pathway]] |
* [[Tuberoinfundibular pathway]] |
||
| + | This innervation explains many of the effects of activating this dopamine system. For instance, the [[mesolimbic pathway]] connects the VTA and [[nucleus accumbens]], both are central to the brain [[reward system]].<ref>[http://www.pdn.cam.ac.uk/staff/schultz/ Schultz, Cambridge university, UK]</ref> |
||
| ⚫ | |||
| + | |||
| + | === Movement === |
||
| + | |||
| + | Via the [[dopamine receptor]]s D1, D2, D3, D4 and D5, dopamine reduces the influence of the indirect pathway, and increases the actions of the direct pathway within the [[basal ganglia]]. Insufficient dopamine [[biosynthesis]] in the dopaminergic neurons can cause [[Parkinson's disease]], in which a person loses the ability to execute smooth, controlled movements. The phasic dopaminergic activation seems to be crucial with respect to a lasting internal encoding of motor skills (Beck, 2005). |
||
| + | |||
| + | === Cognition and frontal cortex === |
||
| + | |||
| ⚫ | In the [[frontal lobe]]s, dopamine controls the flow of information from other areas of the brain. Dopamine disorders in this region of the brain can cause a decline in [[neurocognitive]] functions, especially [[memory]], [[attention]], and [[problem-solving]]. Reduced dopamine concentrations in the prefrontal cortex are thought to contribute to [[attention deficit disorder]]. On the converse, however, [[anti-psychotic]] [[medication]]s act as dopamine antagonists and are used in the treatment of positive symptoms in [[schizophrenia]]. |
||
| + | |||
| ⚫ | |||
| + | |||
| ⚫ | Dopamine is the primary [[neuroendocrine]] regulator of the secretion of [[prolactin]] from the [[anterior pituitary]] gland. Dopamine produced by neurons in the [[arcuate nucleus]] of the hypothalamus is secreted into the hypothalamo-hypophysial blood vessels of the [[median eminence]], which supply the [[pituitary gland]]. The lactotrope cells that produce [[prolactin]], in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion. Thus, in the context of regulating prolactin secretion, dopamine is occasionally called '''prolactin-inhibiting factor''' ('''PIF'''), '''prolactin-inhibiting hormone''' ('''PIH'''), or '''prolactostatin'''. Prolactin also seems to inhibit dopamine release, such as after [[orgasm]], and is chiefly responsible for the [[Refractory period (sex)|refractory period]]. |
||
| + | |||
| + | === Motivation and pleasure === |
||
| + | |||
| + | ==== Reinforcement ==== |
||
| + | |||
| ⚫ | Dopamine is commonly associated with the ''pleasure system'' of the brain, providing feelings of enjoyment and [[reinforcement]] to motivate a person proactively to perform certain activities. Dopamine is released (particularly in areas such as the [[nucleus accumbens]] and [[ventral tegmental area]]) by naturally rewarding experiences such as [[food]], [[Human sexual behaviour|sex]],<ref name="PMID11805404">{{cite journal|last=Giuliano|first=F.|coauthors=Allard J.|year=2001|title=Dopamine and male sexual function|url=|journal=Eur Urol|issn=|volume=40|issue=|pages=601-608|pmid=11805404}}</ref><ref name="PMID11477488">{{cite journal|last=Giuliano|first=F.|coauthors=Allard J. |year=2001|title=Dopamine and sexual function|url=http://www.nature.com/ijir/journal/v13/n3s/index.html|journal=Int J Impot Res|issn=|volume=13|issue=Suppl 3|pages=S18-S28|doi=10.1038/sj.ijir.3900719|pmid=11477488}}</ref> some drugs, and [[neutral stimulus|neutral stimuli]] that become [[Classical conditioning|associated]] with them. This theory is often discussed in terms of drugs such as [[cocaine]], [[nicotine]],and [[amphetamine]]s, which seem to directly or indirectly lead to the increase of dopamine in these areas, and in relation to [[neurobiology|neurobiological]] theories of chemical [[addiction]], arguing that these dopamine pathways are pathologically altered in addicted persons. |
||
| + | |||
| + | ==== Reuptake inhibition, expulsion ==== |
||
| + | |||
| + | However, cocaine and amphetamine influence separate mechanisms of action. Cocaine is a [[dopamine transporter]] blocker that competitively inhibits dopamine uptake to increase the lifetime of dopamine and augments an overabundance of dopamine (an increase of up to 150 percent) within the parameters of the dopamine neurotransmitters. |
||
| + | |||
| + | Like cocaine, amphetamines increase the concentration of dopamine in the [[synapse|synaptic]] gap, but by a different mechanism. Amphetamines are similar in structure to dopamine, and so can enter the terminal button of the presynaptic neuron via its dopamine transporters as well as by diffusing through the [[neural membrane]] directly. When entering inside the presynaptic neuron, amphetamines force the dopamine molecules out of their storage [[vesicle]]s and expel them into the synaptic gap by making the dopamine transporters work in reverse. |
||
| + | |||
| + | ==== Incentive salience ==== |
||
| + | Dopamine's role in experiencing pleasure has been questioned by several researchers. It has been argued that dopamine is more associated with anticipatory desire and motivation (commonly referred to as "wanting") as opposed to actual consummatory pleasure (commonly referred to as "liking"). Dopamine is not released when unpleasant or aversive stimuli are encountered, and so motivates towards the pleasure of avoiding or removing the unpleasant stimuli. |
||
| + | |||
| + | ==== Dopamine, learning, and reward-seeking behavior ==== |
||
| + | Dopaminergic neurons of the midbrain are the main source of dopamine in the brain.<!----><ref name="fn5">{{cite journal | author = Arias-Carrión O, Pöppel E | title = Dopamine, learning and reward-seeking behavior | journal = Act Neurobiol Exp | volume = 67 | issue = 4 | pages = 481-488 | year = 2007 | id = PMID |url=http://www.ane.pl/pdfdownload.php?art=6738 }}</ref> Dopamine has been shown to be involved in the control of movements, the signaling of error in prediction of reward, motivation, and cognition. Cerebral dopamine depletion is the hallmark of Parkinson´s disease.<!----><ref name="fn5">{{cite journal | author = Arias-Carrión O, Freundlieb N, Oertel WH, Höglinger GU | title = Adult neurogenesis and Parkinson's disease | journal = CNS Neurol Disord Drug Targets. | volume = 6 | issue = 5 | pages = 326-335. | year = 2007 | id = PMID 18045161}}</ref> Other pathological states have also been associated with dopamine dysfunction, such as schizophrenia, autism, and attention deficit hyperactivity disorder in children, as well as drug abuse. Dopamine is closely associated with reward-seeking behaviors, such as approach, consumption, and addiction.<!----><ref name="fn5">{{cite journal | author = Arias-Carrión O, Pöppel E | title = Dopamine, learning and reward-seeking behavior | journal = Act Neurobiol Exp | volume = 67 | issue = 4 | pages = 481-488 | year = 2007 | id = PMID }}</ref> Recent researches suggest that the firing of dopaminergic neurons is a motivational substance as a consequence of reward-anticipation. This hypothesis is based on the evidence that, when a reward is greater than expected, the firing of certain dopaminergic neurons increases, which consequently increases desire or motivation towards the reward.<!----><ref name="fn5">{{cite journal | author = Arias-Carrión O, Pöppel E | title = Dopamine, learning and reward-seeking behavior | journal = Act Neurobiol Exp | volume = 67 | issue = 4 | pages = 481-488 | year = 2007 | id = PMID }}</ref> |
||
| + | |||
| + | ==== Animal studies ==== |
||
| + | |||
| + | Clues to dopamine's role in motivation, desire, and pleasure have come from studies performed on animals. In one such study, rats were depleted of dopamine by up to 99 percent in the [[nucleus accumbens]] and [[neostriatum]] using 6-hydroxydopamine.<!-- |
||
| + | --><ref name="fn5">{{cite journal | author = Berridge K, Robinson T | title = What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? | journal = Brain Res Brain Res Rev | volume = 28 | issue = 3 | pages = 309-69 | year = 1998 | id = PMID 9858756}}</ref> |
||
| + | With this large reduction in dopamine, the rats would no longer eat by their own volition. The researchers then force-fed the rats food and noted whether they had the proper facial expressions indicating whether they liked or disliked it. The researchers of this study concluded that the reduction in dopamine did not reduce the rat's consummatory pleasure, only the desire to actually eat. In another study, mutant hyperdopaminergic (increased dopamine) mice show higher "wanting" but not "liking" of sweet rewards.<!-- |
||
| ⚫ | --><ref name="fn1">{{cite journal | author = Peciña S, Cagniard B, Berridge K, Aldridge J, Zhuang X | title = Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. | journal = J Neurosci | volume = 23 | issue = 28 | pages = 9395-402 | year = 2003 | id = PMID 14561867}}</ref> |
||
| + | |||
| + | ==== Dopamine reducing drugs in humans ==== |
||
| + | |||
| + | In humans, however, drugs that reduce dopamine activity (neuroleptics, e.g., some [[antipsychotic]]s) have been shown to reduce motivation, as well as cause [[anhedonia]] (the inability to experience pleasure).<!-- |
||
| ⚫ | --><ref name="fn2">{{cite journal | author = Lambert M, Schimmelmann B, Karow A, Naber D | title = Subjective well-being and initial dysphoric reaction under antipsychotic drugs - concepts, measurement and clinical relevance. | journal = Pharmacopsychiatry | volume = 36 | issue = Suppl 3| pages = S181-90 | year = 2003 | pmid = 14677077}}</ref> |
||
| + | Selective D2/D3 agonists [[pramipexole]] and [[ropinirole]] as used in [[Restless legs syndrome]] have limited anti-anhedonic properties as measured by the Snaith-Hamilton Pleasure Scale.<!-- |
||
| + | --><ref name="fn3">{{cite journal | author = Lemke M, Brecht H, Koester J, Kraus P, Reichmann H | title = Anhedonia, depression, and motor functioning in Parkinson's disease during treatment with pramipexole. | journal = J Neuropsychiatry Clin Neurosci | volume = 17 | issue = 2 | pages = 214-20 | year = 2005 | pmid = 15939976 | url=http://neuro.psychiatryonline.org/cgi/content/full/17/2/214}}</ref> |
||
| + | (The Snaith-Hamilton-Pleasure-Scale (SHAPS), introduced in English in 1995, assesses self-reported anhedonia in psychiatric patients.).These drugs have side effects like "hyper sexuality" and "compulsive gambling" which are pleasure determined effects of dopamine.{{Fact|date=January 2008}} |
||
| + | |||
| ⚫ | |||
| + | |||
| + | ==== Opioid and cannabinoid transmission ==== |
||
| + | |||
| + | [[Opioid]] and [[cannabinoid]] transmission instead of dopamine may modulate consummatory pleasure and food palatability (liking).<!-- |
||
| + | --><ref name="fn4">{{cite journal | author = Peciña S, Berridge K | title = Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? | journal = J Neurosci | volume = 25 | issue = 50 | pages = 11777-86 | year = 2005 | id = PMID 16354936 | url=http://www.jneurosci.org/cgi/content/full/jneuro;25/50/11777}}</ref> |
||
| + | This could explain why animals' "liking" of food is independent of brain dopamine concentration. Other consummatory pleasures, however, may be more associated with dopamine. One study found that both anticipatory and consummatory measures of sexual behavior (male rats) were disrupted by DA receptor antagonists.<!-- |
||
| + | --><ref name="fn6">{{cite journal | author = Pfaus J, Phillips A | title = Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. | journal = Behav Neurosci | volume = 105 | issue = 5 | pages = 727-43 | year = 1991 | id = PMID 1840012}}</ref> |
||
| + | Libido can be increased by drugs that affect dopamine, but not by drugs that affect opioid peptides or other neurotransmitters. |
||
| + | |||
| + | ==== Sociability ==== |
||
| + | |||
| + | Sociability is also closely tied to dopamine neurotransmission. Low D2 receptor-binding is found in people with [[social anxiety]]. Traits common to negative schizophrenia ([[social withdrawal]], [[apathy]], [[anhedonia]]) are thought to be related to a hypodopaminergic state in certain areas of the brain. In instances of [[bipolar disorder]], [[manic]] subjects can become hypersocial, as well as [[hypersexual]]. This is also credited to an increase in dopamine, because mania can be reduced by dopamine-blocking anti-psychotics. |
||
| + | |||
| + | ==== Salience ==== |
||
| + | |||
| + | Dopamine may also have a role in the [[Salience (neuroscience)|salience]] ('noticeableness') of perceived objects and events, with potentially important stimuli such as: 1) rewarding things or 2) dangerous or threatening things seeming more noticeable or important.<ref>{{cite journal | author = Schultz W | title = Getting formal with dopamine and reward | journal = Neuron | volume = 36 | issue = 2 | pages = 241-263 | year = 2002 | id = PMID 12383780}}</ref> This hypothesis argues that dopamine assists decision-making by influencing the priority, or level of desire, of such stimuli to the person concerned. |
||
| + | |||
| + | ==== Behavior disorders ==== |
||
| + | |||
| + | [[Pharmacology|Pharmacological]] blockade of brain dopamine receptors increases rather than decreases drug-taking behaviour. Since blocking dopamine decreases desire, the increase in drug-taking behaviour may be seen as not a chemical desire but as a deeply psychological desire to just 'feel something'. |
||
| + | |||
| + | Deficits in dopamine levels are implicated in [[attention-deficit hyperactivity disorder]] (ADHD), and stimulant medications used to successfully treat the disorder increase dopamine neurotransmitter levels, leading to decreased symptoms. |
||
| + | |||
| + | === Latent inhibition and creative drive === |
||
| + | |||
| + | Dopamine in the [[mesolimbic pathway]] increases general [[arousal]] and goal directed behaviors and decreases [[latent inhibition]]; all three effects increase the creative drive of idea generation. This has led to a three-factor model of [[creativity]] involving the [[frontal lobe]]s, the [[temporal lobe]]s, and mesolimbic dopamine.<ref>{{cite journal | author= Flaherty, A.W, | year = 2005 | journal = Journal of Comparative Neurology | title = Frontotemporal and dopaminergic control of idea generation and creative drive | volume = 493 | issue = 1 | pages = 147-153 | pmid = 16254989}}</ref> |
||
| + | |||
| ⚫ | |||
| + | {{main|Dopamine hypothesis of psychosis}} |
||
| + | |||
| + | Abnormally high dopamine action has also been strongly linked to [[psychosis]] and [[schizophrenia]]<!-- |
||
| + | -->,<ref>{{cite web |publisher=St. Jude Children's Research Hospital |title=Disruption of gene interaction linked to schizophrenia | accessdate=2006-07-06 | url=http://www.innovations-report.com/html/reports/life_sciences/report-52499.html}}</ref> |
||
| + | Dopamine neurons in the [[mesolimbic pathway]] are particularly associated with these conditions. Evidence comes partly from the discovery of a class of drugs called the [[phenothiazine]]s (which block D<sub>2</sub> [[dopamine receptor]]s) that can reduce psychotic symptoms, and partly from the finding that drugs such as [[amphetamine]] and [[cocaine]] (which are known to greatly increase dopamine levels) can cause psychosis.<ref>{{cite journal | last = Lieberman | first = J.A. | coauthors = JM Kane, J. Alvir | title = Provocative tests with psychostimulant drugs in schizophrenia. | journal = Psychopharmacology (Berl). | volume = 91 | issue = 4 | pages = 415-433 | publisher = | date = 1997 | url = http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=pubmed&dopt=AbstractPlus&list_uids=2884687&query_hl=1&itool=pubmed_DocSum | doi = | id = PMID 2884687 | accessdate = 2007-10-16 }}</ref> Because of this, most modern [[antipsychotic]] medications, for example, [[Risperidone]], are designed to block dopamine function to varying degrees. |
||
| + | |||
| ⚫ | |||
| + | {{main|L-DOPA}} |
||
| + | |||
| ⚫ | [[L-DOPA|Levodopa]] is a dopamine precursor used in various forms to treat [[Parkinson's disease]]. It is typically co-administered with an inhibitor of peripheral decarboxylation (DDC, [[Aromatic-L-amino-acid decarboxylase|dopa decarboxylase]]), such as [[carbidopa]] or [[benserazide]]. Inhibitors of alternative metabolic route for dopamine by [[catechol-O-methyl transferase]] are also used. These include [[entacapone]] and [[tolcapone]]. |
||
| + | |||
| ⚫ | |||
| + | |||
| ⚫ | |||
| + | |||
| + | <div style="-moz-column-count:3; column-count: 3;"> |
||
* [[Addiction]] |
* [[Addiction]] |
||
* [[Amphetamine]] |
* [[Amphetamine]] |
||
| Line 186: | Line 267: | ||
* [[Catecholamine]] |
* [[Catecholamine]] |
||
* [[Catechol-O-methyl transferase]] |
* [[Catechol-O-methyl transferase]] |
||
| + | * [[Classical conditioning]] |
||
| + | * [[Operant conditioning]] |
||
* [[Cocaine]] |
* [[Cocaine]] |
||
* [[Dopamine hypothesis of schizophrenia]] |
* [[Dopamine hypothesis of schizophrenia]] |
||
| + | * [[Dopamine metabolites]] |
||
| + | * [[Dopaminergic pathways]] |
||
| + | * [[Dopamine reuptake inhibitor]]s |
||
| + | * [[Heart rate effecting drugs]] |
||
| + | * [[Homovanillic acid]] |
||
| + | * [[Methyldopa]] |
||
| + | * [[Methyphenyltetrahydropyridine]] |
||
* [[Methylphenidate]] |
* [[Methylphenidate]] |
||
| − | * [[ |
+ | * [[Neuromodulation]] |
* [[Parkinson's disease]] |
* [[Parkinson's disease]] |
||
| − | * [[ |
+ | * [[Prolactinoma]] |
| − | * [[Selegiline]] |
+ | * [[Selegiline]]</div> |
| + | * [[Serotonin-dopamine reuptake inhibitor]] |
||
| − | == |
+ | == References == |
| − | {{ |
+ | {{Reflist|2}} |
| − | *[http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=APRD00085 DrugBank APRD00085] |
||
| − | ==References== |
||
| ⚫ | {{ |
||
| ⚫ | {{ |
||
| − | {{fnb|3}} |
||
| − | *[http://neuro.psychiatryonline.org/cgi/content/full/17/2/214 Matthias R. Lemke, M.D., H. Michael Brecht, M.D., Juergen Koester, Ph.D., Peter H. Kraus, M.D. and Heinz Reichmann, M.D.(2005)] Anhedonia, Depression, and Motor Functioning in Parkinson’s Disease During Treatment With Pramipexole.'' J Neuropsychiatry Clin Neurosci''. 17:214-220.<br> |
||
| − | {{fnb|4}}[http://www.jneurosci.org/cgi/content/full/jneuro;25/50/11777 Susana Peciña and Kent C. Berridge .(2005)] Hedonic Hot Spot in Nucleus Accumbens Shell: Where Do µ-Opioids Cause Increased Hedonic Impact of Sweetness?''J Neurosci''. 25(50):11777-11786.<br> |
||
| − | *Pyraki C et al.(1982) Dopaminergic substrates of amphetamine-induced place preference. [[Brain Res.]] 253:185-193, . |
||
| − | *Shippenberg T and Herz A (1988) Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of u- and k-opioid agonists. European. Journal of. Pharmacology. 151: 233-242, . |
||
| + | == External links == |
||
| − | *Wise RA & Rompre PP (1989).Brain dopamine and reward. [[Annual. Review of. Psychology.]] 40: 191-225, |
||
| + | * {{DrugBank|APRD00085}} |
||
| − | *Corrigall WA et al. (1992).The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. [[Psychopharmacology]] 107: 285-289, |
||
| − | *Salamone JD (1994).The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. [[Behav. Brain Res.]] 61: 117-133, |
||
| − | *Pfaus JG et al. (1995) Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. [[Brain Res.]] 693: 21-30, |
||
| − | |||
| − | {{Wiktionarypar|Dopamine}} |
||
{{Phenethylamines}} |
{{Phenethylamines}} |
||
| + | |||
| + | {{Cardiac stimulants excluding cardiac glycosides}} |
||
[[Category:Catecholamines]] |
[[Category:Catecholamines]] |
||
| ⚫ | |||
| + | [[Category:Hormones of the hypothalamus]] |
||
| ⚫ | |||
[[Category:Neurotransmitters]] |
[[Category:Neurotransmitters]] |
||
[[Category:Phenethylamines]] |
[[Category:Phenethylamines]] |
||
| ⚫ | |||
| ⚫ | |||
| − | |||
| + | <!-- |
||
| ⚫ | |||
| + | [[bg:Допамин]] |
||
| ⚫ | |||
| + | [[cs:Dopamin]] |
||
[[da:Dopamin]] |
[[da:Dopamin]] |
||
[[de:Dopamin]] |
[[de:Dopamin]] |
||
| + | [[dv:ޑޯޕަމީން]] |
||
| + | [[et:Dopamiin]] |
||
[[es:Dopamina]] |
[[es:Dopamina]] |
||
[[fr:Dopamine]] |
[[fr:Dopamine]] |
||
| + | [[hr:Dopamin]] |
||
[[it:Dopamina]] |
[[it:Dopamina]] |
||
[[he:דופמין]] |
[[he:דופמין]] |
||
| + | [[lt:Dopaminas]] |
||
| + | [[hu:Dopamin]] |
||
[[nl:Dopamine]] |
[[nl:Dopamine]] |
||
| − | [[ja:ドパミン]] |
+ | [[ja:ドーパミン]] |
| + | [[no:Dopamin]] |
||
[[pl:Dopamina]] |
[[pl:Dopamina]] |
||
[[pt:Dopamina]] |
[[pt:Dopamina]] |
||
[[ru:Дофамин]] |
[[ru:Дофамин]] |
||
| + | [[sr:Допамин]] |
||
[[fi:Dopamiini]] |
[[fi:Dopamiini]] |
||
[[sv:Dopamin]] |
[[sv:Dopamin]] |
||
[[th:โดพามีน]] |
[[th:โดพามีน]] |
||
| + | [[tr:Dopamin]] |
||
[[zh:多巴胺]] |
[[zh:多巴胺]] |
||
| + | --> |
||
| − | |||
{{enWP|Dopamine}} |
{{enWP|Dopamine}} |
||
Latest revision as of 12:20, 22 July 2013
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
| Dopamine | |
|---|---|
| |
| General | |
| Systematic name | 4-(2-aminoethyl)benzene-1,2-diol |
| Other names | 2-(3,4-dihydroxyphenyl)ethylamine; 3,4-dihydroxyphenethylamine; 3-hydroxytyramine; DA; Intropin Revivan; Oxytyramine |
| Molecular formula | C8H11NO2 |
| SMILES | NCCc1ccc(O)c(O)c1 |
| Molar mass | 153.178 g/mol |
| Appearance | white powder with distinctive smell |
| CAS number | [51-61-6] |
| Properties | |
| Density and phase | ? g/cm³, ? |
| Solubility in water | 60.0 g/100 ml (? °C), solid |
| Melting point | 128 °C (401 K) |
| Boiling point | ? °C (? K) |
| Acidity (pKa) | ? |
| Basicity (pKb) | ? |
| Chiral rotation [α]D | ?° |
| Viscosity | ? cP at ? °C |
| Structure | |
| Molecular shape | ? |
| Coordination geometry |
? |
| Crystal structure | ? |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | ? |
| NFPA 704 | ? |
| Flash point | ? °C |
| R/S statement | R: 36/37/38 S: 26-36 |
| RTECS number | UX1088000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | ? |
| Other cations | ? |
| Related | Tyramine, octopamine, norepinephrine (noradrenaline) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Dopamine is a hormone and neurotransmitter occurring in a wide variety of animals, including both vertebrates and invertebrates. In chemical structure, it is a phenethylamine.
In the brain, dopamine functions as a neurotransmitter, activating the five types of dopamine receptors — D1, D2, D3, D4 and D5, and their variants. Dopamine is produced in several areas of the brain, including the substantia nigra. Dopamine is also a neurohormone released by the hypothalamus. Its main function as a hormone is to inhibit the release of prolactin from the anterior lobe of the pituitary.
Dopamine can be supplied as a medication that acts on the sympathetic nervous system, producing effects such as increased heart rate and blood pressure. However, because dopamine cannot cross the blood-brain barrier, dopamine given as a drug does not directly affect the central nervous system. To increase the amount of dopamine in the brains of patients with diseases such as Parkinson's disease and Dopa-Responsive dystonia, L-DOPA (levodopa), which is the precursor of dopamine, can be given because it can cross the blood-brain barrier.
History
Dopamine was discovered by Arvid Carlsson and Nils-Åke Hillarp at the Laboratory for Chemical Pharmacology of the National Heart Institute of Sweden, in 1952. It was named Dopamine because it was a monoamine, and its synthetic precursor was 3,4-dihydroxyphenylalanine (L-DOPA).[1] Arvid Carlsson was awarded the 2000 Nobel Prize in Physiology or Medicine for showing that dopamine is not just a precursor of norepinephrine (noradrenaline) and epinephrine (adrenaline) but a neurotransmitter, as well.
Dopamine was first synthesized artificially in 1910 by George Barger and James Ewens at Wellcome Laboratories in London, England.[2]
Biochemistry
Biosynthesis of Dopamine
Name and family
Dopamine has the chemical formula C6H3(OH)2-CH2-CH2-NH2. Its chemical name is "4-(2-aminoethyl)benzene-1,2-diol" and its abbreviation is "DA."
As a member of the catecholamine family, dopamine is a precursor to norepinephrine (noradrenaline) and then epinephrine (adrenaline) in the biosynthetic pathways for these neurotransmitters.
Biosynthesis
Dopamine is biosynthesized in the body (mainly by nervous tissue and the medulla of the adrenal glands) first by the hydration of the amino acid L-tyrosine to L-DOPA via the enzyme tyrosine 3-monooxygenase, also known as tyrosine hydroxylase, and then by the decarboxylation of L-DOPA by aromatic L-amino acid decarboxylase (which is often referred to as dopa decarboxylase). In some neurons, dopamine is further processed into norepinephrine by dopamine beta-hydroxylase.
In neurons, dopamine is packaged after synthesis into vesicles, which are then released into the synapse in response to a presynaptic action potential.
Inactivation and degradation
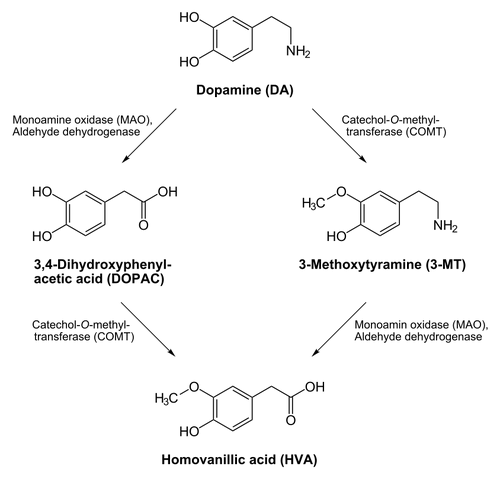
Dopamine is inactivated by reuptake via the dopamine transporter, then enzymatic breakdown by catechol-O-methyl transferase (COMT) and monoamine oxidase (MAO). Dopamine that is not broken down by enzymes is repackaged into vesicles for reuse.
Dopamine may also simply diffuse away from the synapse, and help to regulate blood pressure.
Functions in the brain
Dopamine has many functions in the brain, including important roles in behavior and cognition, motor activity, motivation and reward, regulation of milk production, sleep, mood, attention, and learning. Dopaminergic neurons (i.e., neurons whose primary neurotransmitter is dopamine) are present chiefly in the ventral tegmental area (VTA) of the midbrain, substantia nigra pars compacta, and arcuate nucleus of the hypothalamus.
The phasic responses of dopamine neurons are observed when an unexpected reward is presented. These responses transfer to the onset of a conditioned stimulus after repeated pairings with the reward. Further, dopamine neurons are depressed when the expected reward is omitted. Thus, dopamine neurons seem to encode the prediction error of rewarding outcomes. In nature, we learn to repeat behaviors that lead to maximize rewards. Dopamine is therefore believed to provide a teaching signal to parts of the brain responsible for acquiring new behavior. Temporal difference learning provides a computational model describing how the prediction error of dopamine neurons is used as a teaching signal.
In insects, a similar reward system exists, using octopamine, a chemical relative of dopamine.[3]
Anatomy
Dopaminergic neurons form a neurotransmitter system which originates in substantia nigra pars compacta, ventral tegmental area (VTA) and hypothalamus. These project axons to large areas of the brain through some major pathways:
This innervation explains many of the effects of activating this dopamine system. For instance, the mesolimbic pathway connects the VTA and nucleus accumbens, both are central to the brain reward system.[4]
Movement
Via the dopamine receptors D1, D2, D3, D4 and D5, dopamine reduces the influence of the indirect pathway, and increases the actions of the direct pathway within the basal ganglia. Insufficient dopamine biosynthesis in the dopaminergic neurons can cause Parkinson's disease, in which a person loses the ability to execute smooth, controlled movements. The phasic dopaminergic activation seems to be crucial with respect to a lasting internal encoding of motor skills (Beck, 2005).
Cognition and frontal cortex
In the frontal lobes, dopamine controls the flow of information from other areas of the brain. Dopamine disorders in this region of the brain can cause a decline in neurocognitive functions, especially memory, attention, and problem-solving. Reduced dopamine concentrations in the prefrontal cortex are thought to contribute to attention deficit disorder. On the converse, however, anti-psychotic medications act as dopamine antagonists and are used in the treatment of positive symptoms in schizophrenia.
Regulating prolactin secretion
Dopamine is the primary neuroendocrine regulator of the secretion of prolactin from the anterior pituitary gland. Dopamine produced by neurons in the arcuate nucleus of the hypothalamus is secreted into the hypothalamo-hypophysial blood vessels of the median eminence, which supply the pituitary gland. The lactotrope cells that produce prolactin, in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion. Thus, in the context of regulating prolactin secretion, dopamine is occasionally called prolactin-inhibiting factor (PIF), prolactin-inhibiting hormone (PIH), or prolactostatin. Prolactin also seems to inhibit dopamine release, such as after orgasm, and is chiefly responsible for the refractory period.
Motivation and pleasure
Reinforcement
Dopamine is commonly associated with the pleasure system of the brain, providing feelings of enjoyment and reinforcement to motivate a person proactively to perform certain activities. Dopamine is released (particularly in areas such as the nucleus accumbens and ventral tegmental area) by naturally rewarding experiences such as food, sex,[5][6] some drugs, and neutral stimuli that become associated with them. This theory is often discussed in terms of drugs such as cocaine, nicotine,and amphetamines, which seem to directly or indirectly lead to the increase of dopamine in these areas, and in relation to neurobiological theories of chemical addiction, arguing that these dopamine pathways are pathologically altered in addicted persons.
Reuptake inhibition, expulsion
However, cocaine and amphetamine influence separate mechanisms of action. Cocaine is a dopamine transporter blocker that competitively inhibits dopamine uptake to increase the lifetime of dopamine and augments an overabundance of dopamine (an increase of up to 150 percent) within the parameters of the dopamine neurotransmitters.
Like cocaine, amphetamines increase the concentration of dopamine in the synaptic gap, but by a different mechanism. Amphetamines are similar in structure to dopamine, and so can enter the terminal button of the presynaptic neuron via its dopamine transporters as well as by diffusing through the neural membrane directly. When entering inside the presynaptic neuron, amphetamines force the dopamine molecules out of their storage vesicles and expel them into the synaptic gap by making the dopamine transporters work in reverse.
Incentive salience
Dopamine's role in experiencing pleasure has been questioned by several researchers. It has been argued that dopamine is more associated with anticipatory desire and motivation (commonly referred to as "wanting") as opposed to actual consummatory pleasure (commonly referred to as "liking"). Dopamine is not released when unpleasant or aversive stimuli are encountered, and so motivates towards the pleasure of avoiding or removing the unpleasant stimuli.
Dopamine, learning, and reward-seeking behavior
Dopaminergic neurons of the midbrain are the main source of dopamine in the brain.[7] Dopamine has been shown to be involved in the control of movements, the signaling of error in prediction of reward, motivation, and cognition. Cerebral dopamine depletion is the hallmark of Parkinson´s disease.[7] Other pathological states have also been associated with dopamine dysfunction, such as schizophrenia, autism, and attention deficit hyperactivity disorder in children, as well as drug abuse. Dopamine is closely associated with reward-seeking behaviors, such as approach, consumption, and addiction.[7] Recent researches suggest that the firing of dopaminergic neurons is a motivational substance as a consequence of reward-anticipation. This hypothesis is based on the evidence that, when a reward is greater than expected, the firing of certain dopaminergic neurons increases, which consequently increases desire or motivation towards the reward.[7]
Animal studies
Clues to dopamine's role in motivation, desire, and pleasure have come from studies performed on animals. In one such study, rats were depleted of dopamine by up to 99 percent in the nucleus accumbens and neostriatum using 6-hydroxydopamine.[7] With this large reduction in dopamine, the rats would no longer eat by their own volition. The researchers then force-fed the rats food and noted whether they had the proper facial expressions indicating whether they liked or disliked it. The researchers of this study concluded that the reduction in dopamine did not reduce the rat's consummatory pleasure, only the desire to actually eat. In another study, mutant hyperdopaminergic (increased dopamine) mice show higher "wanting" but not "liking" of sweet rewards.[8]
Dopamine reducing drugs in humans
In humans, however, drugs that reduce dopamine activity (neuroleptics, e.g., some antipsychotics) have been shown to reduce motivation, as well as cause anhedonia (the inability to experience pleasure).[9] Selective D2/D3 agonists pramipexole and ropinirole as used in Restless legs syndrome have limited anti-anhedonic properties as measured by the Snaith-Hamilton Pleasure Scale.[10] (The Snaith-Hamilton-Pleasure-Scale (SHAPS), introduced in English in 1995, assesses self-reported anhedonia in psychiatric patients.).These drugs have side effects like "hyper sexuality" and "compulsive gambling" which are pleasure determined effects of dopamine.[How to reference and link to summary or text]
- Main article: Dopamine antagonists
Opioid and cannabinoid transmission
Opioid and cannabinoid transmission instead of dopamine may modulate consummatory pleasure and food palatability (liking).[11] This could explain why animals' "liking" of food is independent of brain dopamine concentration. Other consummatory pleasures, however, may be more associated with dopamine. One study found that both anticipatory and consummatory measures of sexual behavior (male rats) were disrupted by DA receptor antagonists.[12] Libido can be increased by drugs that affect dopamine, but not by drugs that affect opioid peptides or other neurotransmitters.
Sociability
Sociability is also closely tied to dopamine neurotransmission. Low D2 receptor-binding is found in people with social anxiety. Traits common to negative schizophrenia (social withdrawal, apathy, anhedonia) are thought to be related to a hypodopaminergic state in certain areas of the brain. In instances of bipolar disorder, manic subjects can become hypersocial, as well as hypersexual. This is also credited to an increase in dopamine, because mania can be reduced by dopamine-blocking anti-psychotics.
Salience
Dopamine may also have a role in the salience ('noticeableness') of perceived objects and events, with potentially important stimuli such as: 1) rewarding things or 2) dangerous or threatening things seeming more noticeable or important.[13] This hypothesis argues that dopamine assists decision-making by influencing the priority, or level of desire, of such stimuli to the person concerned.
Behavior disorders
Pharmacological blockade of brain dopamine receptors increases rather than decreases drug-taking behaviour. Since blocking dopamine decreases desire, the increase in drug-taking behaviour may be seen as not a chemical desire but as a deeply psychological desire to just 'feel something'.
Deficits in dopamine levels are implicated in attention-deficit hyperactivity disorder (ADHD), and stimulant medications used to successfully treat the disorder increase dopamine neurotransmitter levels, leading to decreased symptoms.
Latent inhibition and creative drive
Dopamine in the mesolimbic pathway increases general arousal and goal directed behaviors and decreases latent inhibition; all three effects increase the creative drive of idea generation. This has led to a three-factor model of creativity involving the frontal lobes, the temporal lobes, and mesolimbic dopamine.[14]
Links to psychosis
- Main article: Dopamine hypothesis of psychosis
Abnormally high dopamine action has also been strongly linked to psychosis and schizophrenia,[15] Dopamine neurons in the mesolimbic pathway are particularly associated with these conditions. Evidence comes partly from the discovery of a class of drugs called the phenothiazines (which block D2 dopamine receptors) that can reduce psychotic symptoms, and partly from the finding that drugs such as amphetamine and cocaine (which are known to greatly increase dopamine levels) can cause psychosis.[16] Because of this, most modern antipsychotic medications, for example, Risperidone, are designed to block dopamine function to varying degrees.
Therapeutic use
- Main article: L-DOPA
Levodopa is a dopamine precursor used in various forms to treat Parkinson's disease. It is typically co-administered with an inhibitor of peripheral decarboxylation (DDC, dopa decarboxylase), such as carbidopa or benserazide. Inhibitors of alternative metabolic route for dopamine by catechol-O-methyl transferase are also used. These include entacapone and tolcapone.
Dopamine is also used as an inotropic drug in patients with shock to increase cardiac output and blood pressure.
See also
- Addiction
- Amphetamine
- Antipsychotic
- Catecholamine
- Catechol-O-methyl transferase
- Classical conditioning
- Operant conditioning
- Cocaine
- Dopamine hypothesis of schizophrenia
- Dopamine metabolites
- Dopaminergic pathways
- Dopamine reuptake inhibitors
- Heart rate effecting drugs
- Homovanillic acid
- Methyldopa
- Methyphenyltetrahydropyridine
- Methylphenidate
- Neuromodulation
- Parkinson's disease
- Prolactinoma
- Selegiline
References
- ↑ Benes, F.M. Carlsson and the discovery of dopamine. Trends in Pharmacological Sciences, Volume 22, Issue 1, 1 January 2001, Pages 46-47.
- ↑ Fahn, Stanley, "The History of Levodopa as it Pertains to Parkinson’s Disease," Movement Disorder Society’s 10th International Congress of Parkinson's Disease and Movement Disorders on November 1, 2006, in Kyoto, Japan.
- ↑ Barron AB, Maleszka R, Vander Meer RK, Robinson GE (2007). Octopamine modulates honey bee dance behavior. Proc. Natl. Acad. Sci. U.S.A. 104 (5): 1703-7.
- ↑ Schultz, Cambridge university, UK
- ↑ Giuliano, F., Allard J. (2001). Dopamine and male sexual function. Eur Urol 40: 601-608.
- ↑ Giuliano, F., Allard J. (2001). Dopamine and sexual function. Int J Impot Res 13 (Suppl 3): S18-S28.
- ↑ 7.0 7.1 7.2 7.3 7.4 Arias-Carrión O, Pöppel E (2007). Dopamine, learning and reward-seeking behavior. Act Neurobiol Exp 67 (4): 481-488. PMID. Cite error: Invalid
<ref>tag; name "fn5" defined multiple times with different content Cite error: Invalid<ref>tag; name "fn5" defined multiple times with different content Cite error: Invalid<ref>tag; name "fn5" defined multiple times with different content Cite error: Invalid<ref>tag; name "fn5" defined multiple times with different content - ↑ Peciña S, Cagniard B, Berridge K, Aldridge J, Zhuang X (2003). Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards.. J Neurosci 23 (28): 9395-402. PMID 14561867.
- ↑ Lambert M, Schimmelmann B, Karow A, Naber D (2003). Subjective well-being and initial dysphoric reaction under antipsychotic drugs - concepts, measurement and clinical relevance.. Pharmacopsychiatry 36 (Suppl 3): S181-90.
- ↑ Lemke M, Brecht H, Koester J, Kraus P, Reichmann H (2005). Anhedonia, depression, and motor functioning in Parkinson's disease during treatment with pramipexole.. J Neuropsychiatry Clin Neurosci 17 (2): 214-20.
- ↑ Peciña S, Berridge K (2005). Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness?. J Neurosci 25 (50): 11777-86. PMID 16354936.
- ↑ Pfaus J, Phillips A (1991). Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat.. Behav Neurosci 105 (5): 727-43. PMID 1840012.
- ↑ Schultz W (2002). Getting formal with dopamine and reward. Neuron 36 (2): 241-263. PMID 12383780.
- ↑ Flaherty, A.W, (2005). Frontotemporal and dopaminergic control of idea generation and creative drive. Journal of Comparative Neurology 493 (1): 147-153.
- ↑ Disruption of gene interaction linked to schizophrenia. St. Jude Children's Research Hospital. URL accessed on 2006-07-06.
- ↑ Lieberman, J.A., JM Kane, J. Alvir (1997). Provocative tests with psychostimulant drugs in schizophrenia.. Psychopharmacology (Berl). 91 (4): 415-433. PMID 2884687.
External links
{2C-B} {2C-C} {2C-D} {2C-E} {2C-I} {2C-N} {2C-T-2} {2C-T-21} {2C-T-4} {2C-T-7} {2C-T-8} {3C-E} {4-FMP} {Bupropion} {Cathine} {Cathinone} {DESOXY} {Dextroamphetamine} {Methamphetamine} {Diethylcathinone} {Dimethylcathinone} {DOC} {DOB} {DOI} {DOM} {bk-MBDB} {Dopamine} {Br-DFLY} {Ephedrine} {Epinephrine} {Escaline} {Fenfluramine} {Levalbuterol} {Levmetamfetamine} {MBDB} {MDA} {MDMA} {bk-MDMA/MDMC/MDMCat/Methylone} {MDEA} (MDPV) {Mescaline} {Methcathinone} {Methylphenidate} {Norepinephrine} {Phentermine} {Salbutamol} {Tyramine} {Venlafaxine}
Template:Cardiac stimulants excluding cardiac glycosides
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |