No edit summary |
Revision as of 06:54, 9 December 2008
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
Dendrites (from Greek dendron, “tree”) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or soma of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic arbor. Dendrites play a critical role in integrating these synaptic inputs and in determining the extent to which action potentials are produced by the neuron.
Morphology
Dendrites are made up of dendritic segments which are named according to their relative position to the soma. For example, the initial segment of a dendrite that projects directly from a neuron cell body is called a “first order” segment. When a first order segment branches, its daughter segments are considered “second order” segments. Likewise, daughter segments of second order segments are called “third order” segments. This naming process continues until a dendrite segment ceases to branch. Non-branching dendrites are termed “terminal segments”. In addition, many dendrites contain dendritic spines, small mushroom like protrusions that offer isolated computational compartments on which axon terminals can create synapses.
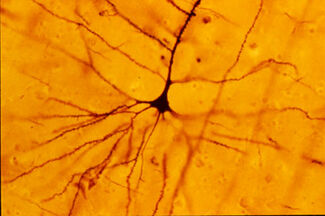
A human neocortical pyramidal neuron stained via Golgi technique. Notice the apical dendrite extending vertically above the soma and the numerous basal dendrites radiating laterally from the base of the cell body.
In general, whereas axons may span the length of nearly a meter, dendrites are more localised around the cell body. Two examples are spinal motor neurons with dendritic arbors of a few millimetres in diameter, and retinal amacrine cells, with arbors of only few hundred micrometers in diameter. Dendrites account for the bulk of the surface area of neurons, with as much as 98% of the neuronal phospholipid bilayer membrane devoted to the dendritic arbor. Additionally, excitatory and inhibitory synapses distribute themselves on the dendritic arbor such that excitatory synapses usually occupy more distal locations while inhibitory synapses are typically located more proximally.
Dendritic morphology, the structural and physical architecture of the dendritic arbor, varies greatly across neuronal type. Dendrites of Purkinje cells, a dominant cell type in the cerebellum, extend from the soma to form a nearly two dimensional arborization. Purkinje cells are stacked in the cerebellum much like dominoes (i.e., with their dendritic arbors parallel to each other) allowing the afferent connections of parallel fibers to run orthogonally through the Purkinje cell dendrites. Pyramidal neurons, the most prevalent excitatory neuron in the cerebral cortex harbour two different classes of dendrites. The apical dendrites extend vertically from the apex of the pyramidally shaped soma and project toward the pial surface of the cerebral hemispheres. The basal dendrites of pyramidal neurons project laterally from the base of the soma. The distinction between different dendrite classes is particularly important for pyramidal neurons as apical dendrites typically receive afferent input from distant sources such as the thalamus while the basal dendrites receive inputs from nearby cortical areas.

A sketch of a cerebellar Purkinje cell drawn by Santiago Ramon y Cajal highlighting the dendrites' intricate branching pattern and two dimensional architecture.
Although a neuron’s general dendrite architecture (such as the two dimensional architecture of Purkinje cell dendrites) is conserved across all areas of the brain in which they may be located, some measures of a neuron’s dendritic architecture may vary significantly according to its location in the brain. Such measures include the total dendritic length (the linear sum of all dendrite segments), the average number of dendrite segments per neuron, and the total number of dendritic spines per dendritic arbor. Pyramidal cell basal dendrites offer a prime example of dendritic morphology across brain area. Pyramidal neurons located in the caudal portions of the cerebral cortex or in primary sensory areas contain basal dendrites which are shorter in total length, have fewer segments, and contain fewer dendritic spines than pyramidal neurons in rostral areas or in association cortices. For example, pyramidal cell basal dendrites in the primary visual cortex are, on average, shorter, less segmented, and contain fewer spines than basal dendrites in visual area MT, which in turn are shorter, less segmented, and contain fewer spines than basal dendrites in prefrontal cortex. In this light, a correlation can be drawn relating the morphologic complexity of a dendritic arbor and the functional complexity of the area in which the neuron is embedded.
Electrical properties of dendrites
The structure and branching of a neuron's dendrites, as well as the availability and variation in voltage-gated ion conductances, strongly influences how it integrates the input from other neurons, particularly those that input only weakly. This integration is both "temporal" -- involving the summation of stimuli that arrive in rapid succession -- as well as "spatial" -- entailing the aggregation of excitatory and inhibitory inputs from separate branches.
Dendrites were once believed to merely convey stimulation passively. In this example voltage changes measured at the cell body result from activations of distal synapses propagating to the soma without the aid of voltage-gated ion channels. Passive cable theory, which descends from linear cable theory, describes how voltage changes at a particular location on a dendrite transmit this electrical signal through a system of converging dendrite segments of different diameters, lengths, and electrical properties. Based on passive cable theory one can track how changes in a neuron’s dendritic morphology changes the membrane voltage at the soma, and thus how variation in dendrite architectures affects the overall output characteristics of the neuron.
Although passive cable theory offers insights regarding input propagation along dendrite segments, it is important to remember that dendrite membranes are host to a cornucopia of proteins some of which may help amplify or attenuate synaptic input. Sodium, calcium, and potassium channels are all implicated in contributing to input modulation. It is possible that each of these ion species has a family of channel types each with its own biophysical characteristics relevant to synaptic input modulation. Such characteristics include the latency of channel opening, the electrical conductance of the ion pore, the activation voltage, and the activation duration. In this way, a weak input from a distal synapse can be amplified by sodium and calcium currents inroute to the soma so that the effects of distal synapse are no less robust than those of a proximal synapse.
One important feature of dendrites, endowed by their active voltage gated conductances, is their ability to send action potentials back into the dendritic arbor. Known as backpropagating action potentials, these signals depolarize the dendritic arbor and provide a crucial component toward synapse modulation and long-term potentiation.
Dendrite development
Despite the critical role that dendrites play in the computational tendencies of neurons, very little is known about the process by which dendrites orient themselves in vivo and are compelled to create the intricate branching pattern unique to each specific neuronal class. It is likely that a complex array of extracellular and intracellular cues modulate dendrite development. Early candidates include: Sema3A, Notch, CREST, and Dasm1. Sema3A may act as a dendritic chemoattractant that aids cortical pyramidal neurons in orienting their apical dendrites to the pial surface. Notch acts as a neurotrophic factor in aiding dendrite growth and branching, while CREST may play an important role in regulating calcium dependant growth signals. Dasm1 (Dendrite arborization and synapse maturation 1) expression appears to be highly localized to dendrites and may have substantial influence on dendrite (but not axon) development.
See also
References
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science, 4th ed. McGraw-Hill, New York (2000). ISBN 0838577016
- Koch C. Biophysics of Computation, Oxford University Press, Oxford (1999). ISBN 0195104919
- Stuart G, Spruston N, Hausser M. Dendrites, Oxford University Press, USA (2000). ISBN 019850488
da:Dendrit de:Dendrit (Biologie) es:Dendrita fa:دندریت fr:Dendrite (biologie) is:Griplur he:דנדריט lt:Dendritas mk:Дендрит nl:Dendriet pt:Dendritos ro:Dendrită ru:Дендрит sv:Dendrit
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |