Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
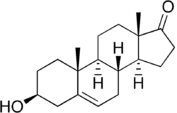
| (3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one IUPAC name | |
| CAS number 53-43-0 |
ATC code |
| PubChem 5881 |
DrugBank DB01708 |
| Chemical formula | {{{chemical_formula}}} |
| Molecular weight | 288.424 g/mol |
| Bioavailability | {{{bioavailability}}} |
| Metabolism | Hepatic |
| Elimination half-life | 12 hours |
| Excretion | Urinary:?% |
| Pregnancy category | {{{pregnancy_category}}} |
| Legal status | {{{legal_status}}} |
| Routes of administration | Oral |
Dehydroepiandrosterone (DHEA, more correctly didehydroepiandrosterone; brand name Ovaflo, Fidelin, Ovomax), also known as androstenolone or prasterone (INN), as well as 3β-hydroxyandrost-5-en-17-one or 5-androsten-3β-ol-17-one, is an important endogenous steroid hormone.[1] It is the most abundant circulating steroid in humans,[2] in whom it is produced in the adrenal glands,[3] the gonads, and the brain,[4] where it functions predominantly as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids.[1][5] However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors,[6] and acting as a neurosteroid.[7]
Effects and uses[]
In women with adrenal insufficiency and the healthy elderly there is insufficient evidence to support the use of DHEA.[8][9]
Strength[]
Evidence is inconclusive in regards to the effect of DHEA on strength in the elderly.[10]
In middle-aged men, no statistically significant effect of DHEA supplementation on lean body mass, strength, or testosterone levels was found in a randomized placebo-controlled trial.[11] One large (100 subjects) trial found no effect on strength following DHEA supplementation in the elderly group in the study.[12]
However, a small study suggested DHEA supplementation was associated with increases in free (but not total) testosterone levels.[13]
In postmenopausal women, within a randomized placebo-controlled trial, no statistically significant effect of DHEA supplementation on muscle strength during a 12 week combined endurance and weight training program.[14][15]
Memory[]
DHEA supplementation has not been found to be useful for memory function in normal middle aged or older adults.[16] It has been studied as a treatment for Alzheimer's disease, but there is no evidence that it is effective.[17]
Female reproductive health[]
Since 2000, DHEA supplementation has been used in reproductive medicine in combination with gonadotropins as a way to treat female infertility.[18]
Cardiovascular disease and risk of death[]
A review in 2003 found the then-extant evidence sufficient to suggest that low serum levels of DHEAS may be associated with coronary heart disease in men, but insufficient to determine whether DHEA supplementation would have any cardiovascular benefit.[19]
A 1986 study found that a higher level of endogenous DHEA, as determined by a single measurement, correlated with a lower risk of death or cardiovascular disease.[20] However, a 2006 study found no correlation between DHEA levels and risk of cardiovascular disease or death in men.[21] A 2007 study found that DHEA restored oxidative balance in diabetic patients, reducing tissue levels of pentosidine—a biomarker for advanced glycation endproducts.[22]
Lupus[]
There is some evidence of short term benefit in those with systemic lupus erythematosus but little evidence of long term benefit or safety.[23]
Safety[]
DHEA is produced naturally in the human body, but the long term effects of its use are largely unknown.[24][25] In the short term, several studies have noted few adverse effects. In a study by Chang et al., DHEA was administered at a dose of 200 mg/day for 24 weeks with slight androgenic effects noted.[26] Another study utilized a dose up to 400 mg/day for 8 weeks with few adverse events reported.[27] A longer term study followed patients dosed with 50 mg of DHEA for 12 months with the number and severity of side effects reported to be small.[28] Another study delivered a dose of 50 mg of DHEA for 10 months with no serious adverse events reported.[29]
As a hormone precursor, there has been a smattering of reports of side effects possibly caused by the hormone metabolites of DHEA.[25][30]
It is not known whether DHEA is safe for long-term use. Some researchers believe DHEA supplements might actually raise the risk of breast cancer, prostate cancer, heart disease, diabetes,[25] and stroke. DHEA may stimulate tumor growth in types of cancer that are sensitive to hormones, such as some types of breast, uterine, and prostate cancer.[25] DHEA may increase prostate swelling in men with benign prostatic hyperplasia (BPH), an enlarged prostate gland.[24]
DHEA is a steroid hormone. High doses may cause aggressiveness, irritability, trouble sleeping, and the growth of body or facial hair on women.[24] It also may stop menstruation and lower the levels of HDL ("good" cholesterol), which could raise the risk of heart disease.[24] Other reported side effects include acne, heart rhythm problems, liver problems, hair loss (from the scalp), and oily skin. It may also alter the body's regulation of blood sugar.[24]
DHEA should not be used with tamoxifen, as it may promote tamoxifen resistance.[24] Patients on hormone replacement therapy may have more estrogen-related side effects when taking DHEA. This supplement may also interfere with other medicines, and potential interactions between it and drugs and herbs should be considered. Always tell your doctor and pharmacist about any supplements and herbs you are taking.[24]
DHEA is possibly unsafe for individuals experiencing the following conditions: pregnancy and breast-feeding, hormone sensitive conditions, liver problems, diabetes, depression or mood disorders, polycystic ovarian syndrome (PCOS), or cholesterol problems.[31] Individuals experiencing any of these conditions should consult with a doctor before taking.
Dehydroepiandrosterone sulfate[]
- Main article: Dehydroepiandrosterone sulfate
Dehydroepiandrosterone sulfate (DHEAS) is the sulfate ester of DHEA. This conversion is reversibly catalyzed by sulfotransferase (SULT2A1) primarily in the adrenals, the liver, and small intestine. In the blood, most DHEA is found as DHEAS with levels that are about 300 times higher than those of free DHEA. Orally ingested DHEA is converted to its sulfate when passing through intestines and liver. Whereas DHEA levels naturally reach their peak in the early morning hours, DHEAS levels show no diurnal variation. From a practical point of view, measurement of DHEAS is preferable to DHEA, as levels are more stable.[citation needed]
Production[]
Comprehensive overview of steroidogenesis, showing DHEA at left among the androgens.
DHEA is produced from cholesterol through two cytochrome P450 enzymes. Cholesterol is converted to pregnenolone by the enzyme P450 scc (side chain cleavage); then another enzyme, CYP17A1, converts pregnenolone to 17α-hydroxypregnenolone and then to DHEA.[32]
Mechanism of action[]
Although it predominantly functions as an endogenous precursor to more potent androgens such as testosterone and DHT, DHEA has been found to possess some degree of androgenic activity in its own right, acting as a low affinity (Ki = 1 μM), weak partial agonist of the androgen receptor. However, its intrinsic activity at the receptor is almost completely negligible, and on account of that, due to competition for binding with full agonists like testosterone, it actually behaves much more like an antagonist there, and hence, like an antiandrogen. However, its affinity for the receptor is very low, and for that reason, is unlikely to be of any significance under normal circumstances.[33][34]
In addition to its affinity for the androgen receptor, DHEA has also been found to bind to and activate the ERα and ERβ estrogen receptors with Ki values of 1.1 μM and 0.5 μM, respectively, and EC50 values of >1 μM and 200 nM, respectively. Though it was found to be a partial agonist of the ERα with a maximal efficacy of 30-70%, the concentrations required for this degree of activation make it unlikely that the activity of DHEA at this receptor is physiologically meaningful. Remarkably however, DHEA acts as a full agonist of the ERβ with a maximal response similar to or actually slightly greater than that of estradiol, and its levels in circulation and local tissues in the human body are high enough to activate the receptor to the same degree as that seen with circulating estradiol levels at somewhat higher than their maximal, non-ovulatory concentrations; indeed, when combined with estradiol with both at levels equivalent to those of their physiological concentrations, overall activation of the ERβ was doubled. As such, it has been proposed that DHEA may be an important and potentially major endogenous estrogen in the body.[6][33]
Unlike the case of the androgen and estrogen receptors, DHEA does not bind to or activate the progesterone, glucocorticoid, or mineralocorticoid receptors.[33][35]
Other nuclear receptor targets of DHEA include the PPARα, PXR, and CAR. In addition, it has been found to directly act on several membrane receptors, including the NMDA receptor as a positive allosteric modulator, the GABAA receptor as a negative allosteric modulator, and the σ1 receptor as an agonist. It is these actions that have conferred the label of a "neurosteroid" upon DHEA. Finally, DHEA is thought to regulate a handful of other proteins via indirect, genomic mechanisms, including the enzymes P4502C11 and 11β-HSD1—the latter of which is essential for the biosynthesis of the glucocorticoids such as cortisol and has been suggested to be involved in the antiglucocorticoid effects of DHEA—and the carrier IGFBP1.[33][36]
Measurement[]
As almost all DHEA is derived from the adrenal glands, blood measurements of DHEAS/DHEA are useful to detect excess adrenal activity as seen in adrenal cancer or hyperplasia, including certain forms of congenital adrenal hyperplasia. Women with polycystic ovary syndrome tend to have elevated levels of DHEAS.[37]
Increasing endogenous production[]
Regular exercise is known to increase DHEA production in the body.[38][39] Calorie restriction has also been shown to increase DHEA in primates.[40] Some theorize that the increase in endogenous DHEA brought about by calorie restriction is partially responsible for the longer life expectancy known to be associated with calorie restriction.[41]
Isomers[]
The term "dehydroepiandrosterone" is ambiguous chemically because it does not include the specific positions within epiandrosterone at which hydrogen atoms are missing. DHEA has a number of naturally occurring isomers that may have similar pharmacological effects. Some isomers of DHEA are 1-dehydroepiandrosterone and 4-dehydroepiandrosterone. These isomers are also technically DHEA, since they are dehydroepiandrosterones in which hydrogens are removed from the epiandrosterone skeleton.
Society and culture[]
Legality[]
United States[]
DHEA is legal to sell in the United States as a dietary supplement. It is currently grandfathered in as an "Old Dietary Ingredient" being on sale prior to 1994. DHEA is specifically exempted from the Anabolic Steroid Control Act of 1990 and 2004[42] It is banned from use in athletic competition.
Canada[]
In Canada, a prescription is required to buy DHEA.[43]Template:Coglin
Australia[]
In Australia, a prescription is required to buy DHEA, where it is also comparatively expensive compared to off-the-shelf purchases in US supplement shops. Australian customs classify DHEA as an "anabolic steroid[s] or precursor[s]" and, as such, it is only possible to carry DHEA into the country through customs if one possesses an import permit which may be obtained if one has a valid prescription for the hormone. [44]
Sports and athletics[]
DHEA is a prohibited substance under the World Anti-Doping Code of the World Anti-Doping Agency,[45] which manages drug testing for Olympics and other sports. In January 2011, NBA player O.J. Mayo was given a 10-game suspension after testing positive for DHEA. Mayo termed his use of DHEA as "an honest mistake".[46] Mayo is the seventh player to test positive for performance-enhancing drugs since the league began testing in 1999. Rashard Lewis, then with the Orlando Magic, tested positive for DHEA and was suspended 10 games before the start of the 2009-10 season.[47] 2008 Olympic 400 meter champion Lashawn Merritt has also tested positive for DHEA and was banned from the sport for 21 months.[48]
Marketing[]
In the United States, DHEA or DHEAS have been advertised with exaggerated claims that they may be beneficial for a wide variety of ailments. DHEA and DHEAS are readily available in the United States, where they are marketed as over-the-counter dietary supplements.[49] A 2004 review in the American Journal of Sports Medicine concluded that "The marketing of this supplement's effectiveness far exceeds its science."[50]
Research[]
Cancer[]
Some in vitro studies have found DHEA to have both antiproliferative and apoptotic effect on cancer cell lines.[51][52][53] The clinical significance of these findings, if any, is unknown. Higher levels of DHEA and other endogenous sex hormones are strongly associated with an increased risk of developing breast cancer in both pre- and postmenopausal women.[54][55]
See also[]
References[]
- ↑ 1.0 1.1 Mo Q, Lu SF, Simon NG (April 2006). Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity. J. Steroid Biochem. Mol. Biol. 99 (1): 50–8.
- ↑ William F Ganong MD, 'Review of Medical Physiology', 22nd Ed, McGraw Hill, 2005, page 362.
- ↑ The Merck Index, 13th Edition, 7798
- ↑ Schulman, Robert A., M.D.; Dean, Carolyn, M.D. (2007). Solve It With Supplements, New York City: Rodale, Inc.. "DHEA (Dehydroepiandrosterone) is a common hormone produced in the adrenal glands, the gonads, and the brain."
- ↑ Thomas Scott (1996). Concise Encyclopedia Biology, Walter de Gruyter. URL accessed 25 May 2012.
- ↑ 6.0 6.1 Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK (2006). The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metabolism Reviews 38 (1–2): 89–116.
- ↑ Friess E, Schiffelholz T, Steckler T, Steiger A (December 2000). Dehydroepiandrosterone--a neurosteroid. European Journal of Clinical Investigation 30 Suppl 3: 46–50.
- ↑ Arlt, W (2004 Sep). Dehydroepiandrosterone and ageing. Best practice & research. Clinical endocrinology & metabolism 18 (3): 363–80.
- ↑ Alkatib, AA, Cosma, M, Elamin, MB, Erickson, D, Swiglo, BA, Erwin, PJ, Montori, VM (2009 Oct). A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. The Journal of Clinical Endocrinology and Metabolism 94 (10): 3676–81.
- ↑ Baker, WL, Karan, S, Kenny, AM (2011 Jun). Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. Journal of the American Geriatrics Society 59 (6): 997–1002.
- ↑ Wallace, M. B.; Lim, J.; Cutler, A.; Bucci, L. (1999). Effects of dehydroepiandrosterone vs androstenedione supplementation in men. Medicine and Science in Sports and Exercise 31 (12): 1788–92.
- ↑ Muller M;van den Beld AW;van der Schouw YT;Grobbee DE;Lamberts SW. (2006). Effects of dehydroepiandrosterone and atamestane supplementation on frailty in elderly men.. Journal of Clinical Endocrinology 91 (10).
- ↑ Liu et al. (2013). Effect of acute DHEA administration on free testosterone in middle-aged and young men following high-intensity interval training.. Eur J Appl Physiol..
- ↑ Igwebuike, Ada, et al (2008). Lack of DHEA effect on a combined endurance and resistance exercise program in postmenopausal women. Journal of Clinical Endocrinology and Metabolism 95 (12): 534–538.
- ↑ Brainum, J. (2009). DHEA Fountain of Youth or Washout. Iron Man Magazine: 534–538.
- ↑ Grimley Evans, J, Malouf, R, Huppert, F, van Niekerk, JK (2006 Oct 18). Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane database of systematic reviews (Online) (4): CD006221.
- ↑ Fuller, SJ, Tan, RS, Martins, RN (2007 Sep). Androgens in the etiology of Alzheimer's disease in aging men and possible therapeutic interventions. Journal of Alzheimer's disease : JAD 12 (2): 129–42.
- ↑ Casson PR et al. (2000). Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod 15 (10): 2129–2132.
- ↑ Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA (October 2003). Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases? A review of prospective and retrospective studies. Acta Cardiol 58 (5): 403–10.
- ↑ Barrett-Connor, E.; Khaw, K. T.; Yen, S. S. (1986). A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N. Engl. J. Med. 315 (24): 1519–24.
- ↑ Arnlöv, J.; Pencina, M. J.; Amin, S. et al. (2006). Endogenous sex hormones and cardiovascular disease incidence in men. Ann. Intern. Med. 145 (3): 176–84.
- ↑ Boggs, Will. DHEA Restores Oxidative Balance in Type 2 Diabetes. Medscape. URL accessed on 2007-12-14.
- ↑ Crosbie, D, Black, C, McIntyre, L, Royle, PL, Thomas, S (2007 Oct 17). Dehydroepiandrosterone for systemic lupus erythematosus. Cochrane database of systematic reviews (Online) (4): CD005114.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 DHEA. Cancer.org. URL accessed on 24 July 2012.
- ↑ 25.0 25.1 25.2 25.3 Medscape (2010). DHEA Oral. Drug Reference. WebMD LLC.. URL accessed on 18 February 2010.
- ↑ Chang DM, Lan JL, Lin HY, Luo SF (2002). Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum 46 (11): 2924–2927.
- ↑ Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ, Ferrando SJ (2006). Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry. 163 (1): 59–66.
- ↑ Brooke AM, Kalingag LA, Miraki-Moud F, Camacho-Hübner C, Maher KT, Walker DM, Hinson JP, Monson JP (2006). Dehydroepiandrosterone improves psychological well-being in male and female hypopituitary patients on maintenance growth hormone replacement. J Clin Endocrinol Metab 91 (10): 3773–3779.
- ↑ Villareal DT, Holloszy JO (2006). DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab 291 (5): E1003–1008.
- ↑ Medline Plus DHEA. Drugs and Supplements Information. National Library of Medicine. URL accessed on 18 February 2010.
- ↑ DHEA: Side effects and safety. WebMD. URL accessed on 24 July 2012.
- ↑ Harper's illustrated Biochemistry, 27th edition, Ch.41 "The Diversity of the Endocrine system"
- ↑ 33.0 33.1 33.2 33.3 Chen F, Knecht K, Birzin E, et al. (November 2005). Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology 146 (11): 4568–76.
- ↑ Gao W, Bohl CE, Dalton JT (September 2005). Chemistry and structural biology of androgen receptor. Chemical Reviews 105 (9): 3352–70.
- ↑ Lindschau C, Kirsch T, Klinge U, Kolkhof P, Peters I, Fiebeler A (September 2011). Dehydroepiandrosterone-induced phosphorylation and translocation of FoxO1 depend on the mineralocorticoid receptor. Hypertension 58 (3): 471–8.
- ↑ Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W (February 1994). Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Molecular and Cellular Biochemistry 131 (2): 99–104.
- ↑ Banaszewska B, Spaczyński RZ, Pelesz M, Pawelczyk L (2003). Incidence of elevated LH/FSH ratioin polycystic ovary syndrome women with normo- and hyperinsulinemia. Annales Academiae Medicae Bialostocensis 48.
- ↑ (1998). Effects of amount of training on the saliva concentrations of cortisol, dehydroepiandrosterone and on the dehydroepiandrosterone: Cortisol concentration ratio in women over 16 weeks of training. Eur. J. Appl. Physiol. Occup. Physiol. 78 (5): 466–471.
- ↑ (2002). Hormonal Responses to Endurance and Resistance Exercise in Females Aged 19-69 Years. J. Gerontol. A. Biol. Sci. Med. Sci. 57 (4): B158–165.
- ↑ Mattison, Julie A. (2003). Calorie restriction in rhesus monkeys. Experimental Gerontology 38 (1–2): 35–46..
- ↑ Roberts, E. (1999). The importance of dehydroepiandrosterone sulfate in the blood of primates: a longer and healthier life?. Biochemical Pharmacology 57 (4): 329–346..
- ↑ Drug Scheduling Actions - 2005. Drug Enforcement Administration.
- ↑ Dr. Michael Colgin. The Deal With D.H.E.A.. Vista Magazine Online. vistamag.com.
- ↑ Therapeutic Goods Administration, Personal Importation Scheme
- ↑ World Anti-Doping Agency
- ↑ Memphis Grizzlies' O.J. Mayo gets 10-game drug suspension, ESPN, January 27, 2011.
- ↑ Memphis Grizzlies' O.J. Mayo suspended 10 games for violating NBA anti-drug program
- ↑ US 400m star LaShawn Merritt fails drug test. BBC Sport.
- ↑ Calfee, R.; Fadale, P. (March 2006). Popular ergogenic drugs and supplements in young athletes. Pediatrics 117 (3): e577–89.
- ↑ Tokish, J. M.; Kocher, M. S.; Hawkins, R. J. (2004). Ergogenic aids: a review of basic science, performance, side effects, and status in sports. The American Journal of Sports Medicine 32 (6): 1543–53.
- ↑ Yang, N. C.; Jeng, K. C.; Ho, W. M.; Hu, M. L. (2002). ATP depletion is an important factor in DHEA-induced growth inhibition and apoptosis in BV-2 cells. Life Sci. 70 (17): 1979–88.
- ↑ Schulz, S.; Klann, R. C.; Schönfeld, S.; Nyce, J. W. (1992). Mechanisms of cell growth inhibition and cell cycle arrest in human colonic adenocarcinoma cells by dehydroepiandrosterone: role of isoprenoid biosynthesis. Cancer Res. 52 (5): 1372–6.
- ↑ Loria, R. M. (2002). Immune up-regulation and tumor apoptosis by androstene steroids. Steroids 67 (12): 953–66.
- ↑ Tworoger, S. S.; Missmer, S. A.; Eliassen, A. H. et al. (2006). The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol. Biomarkers Prev. 15 (5): 967–71.
- ↑ Key, T.; Appleby, P.; Barnes, I.; Reeves, G. (2002). Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl. Cancer Inst. 94 (8): 606–16.
External links[]
- Information on DHEA from the Mayo Clinic
- DHEA in elderly women and DHEA or testosterone in elderly men, published in the New England Journal of Medicine in 2006.
Anabolic steroids (A14) (trademark names in brackets) | |
|---|---|
| Androstan (carbon 19 present) |
Androstadienone • Boldenone undecylenate (Equipoise) • Desoxymethyltestosterone (Madol) • DHT • Methandrostenolone (Dianabol) • Methenolone • Norethandrolone • Oxandrolone (Anavar) • Oxymetholone (Anadrol) • Quinbolone (Anabolicum Vister) • Stanozolol (Winstrol) • Testosterone • Clostebol • 4-Chlordehydromethyltestosterone (Turinabol) • Fluoxymesterone (Halotestin) • Drostanolone (Masteron) • DHEA • Oxymetholone (Anadrol-50) • Mesterolone (Proviron) • Methenolone enanthate (Primobolan) • Mestanolone |
| Estren (carbon 19 absent) |
Ethylestrenol • Nandrolone (Deca Durabolin) • Norbolethone (Genabol) • Oxabolone cipionate • Trenbolone (Fina) • Mibolerone (Cheque Drops) • Tetrahydrogestrinone (The Clear) |
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |