No edit summary |
(→History: deleted) |
||
| Line 5: | Line 5: | ||
In Chinese, the symbol for the "catalyst" is the same as for "marriage broker" - which is exactly how catalysts can be thought of: a substance that brings molecules together in a reaction without getting involved in the reaction, or marriage, and can be used repeatedly without affecting the overall reaction. |
In Chinese, the symbol for the "catalyst" is the same as for "marriage broker" - which is exactly how catalysts can be thought of: a substance that brings molecules together in a reaction without getting involved in the reaction, or marriage, and can be used repeatedly without affecting the overall reaction. |
||
| − | ==History== |
||
| − | The phrase ''catalysis'' was coined by [[Jöns Jakob Berzelius]] in 1835 who was the first to note that certain chemicals speed up a reaction. Other early chemists involved in catalysis were [[Alexander Mitscherlich]] who in 1831 referred to ''contact processes'' and [[Johann Wolfgang Döbereiner]] who spoke of ''contact action'' and whose [[Lighter (fire starter)|lighter]] based on [[hydrogen]] and a [[platinum]] sponge became a huge commercial success in the 1820’s. |
||
==Importance of Catalysis== |
==Importance of Catalysis== |
||
Revision as of 22:41, 21 December 2008
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
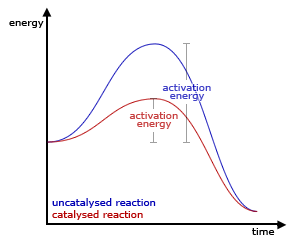
Generic graph showing the effect of a catalyst in an hypothetical exothermic chemical reaction. Notice that the catalysed (red) pathway, despite having a lower activation energy, produces the same final result.
In chemistry and biology, catalysis is the acceleration (increase in rate) of a chemical reaction by means of a substance, called a catalyst, that is itself not consumed by the overall reaction. The word is derived from the Greek noun κατάλυσις, related to the verb καταλύειν, meaning to annul or to untie or to pick up.
In Chinese, the symbol for the "catalyst" is the same as for "marriage broker" - which is exactly how catalysts can be thought of: a substance that brings molecules together in a reaction without getting involved in the reaction, or marriage, and can be used repeatedly without affecting the overall reaction.
Importance of Catalysis
Catalysis is a very important process from an industrial point of view since the production of most industrially important chemicals involve catalysis. The earliest commercial processes are the Haber process for ammonia synthesis and the Fischer-Tropsch synthesis. Research into catalysis is a major field in applied science, and involves many fields of chemistry, notably in organometallic chemistry, and physics. Catalysis is important in many aspects of environmental science, from the catalytic converter in automobiles to the causes of the ozone hole.
Catalytic processes
- Acid-base catalysis
- Catalytic converters made from platinum and rhodium break down some of the more harmful byproducts of automobile exhaust.
- Fuel cells
- Fischer-Tropsch synthesis.
- Haber process (synthesis of ammonia from nitrogen and hydrogen, where ordinary iron is used as a catalyst)
- Hydrogenation
- Methanol synthesis
- Nitric acid production
- Petroleum refining and processing
- Alkylation
- Catalytic cracking - breaking long-chain hydrocarbons into smaller pieces
- Naphtha reforming
- Steam reforming of hydrocarbons to produce synthesis gas
- Sulfuric acid production
- Transesterification
- Olefin polymerisation
See also
- Catalyst
- Autocatalysis
External links
- W.A. Herrmann Technische Universität presentation [1]
- University of York catalyst pages [2]
de:Katalyse es:Catálisis fr:Catalyse nl:Katalyse pt:Catálise sk:Katalýza zh:催化
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |