(avoid display garbage from chinese marks) Tag: rte-source |
m (Reverted edits by 83.29.33.9 (talk | block) to last version by 108.20.46.68) |
||
| Line 1: | Line 1: | ||
{{ClinPsy}} |
{{ClinPsy}} |
||
{{DiseaseDisorder infobox | |
{{DiseaseDisorder infobox | |
||
| − | + | Name = Canavan disease | |
|
| − | + | ICD10 = | |
|
| − | + | ICD9 = {{ICD9|330.0}} | |
|
}} |
}} |
||
'''Canavan disease''' is an inherited disorder that causes progressive damage to [[nerve cell]]s in the [[brain]]. This disease is one of a group of genetic disorders called [[leukodystrophies]].Leukodystrophies are characterized by degeneration of [[myelin]], which is the [[phospholipid]] layer covering that insulates nerve fibers. |
'''Canavan disease''' is an inherited disorder that causes progressive damage to [[nerve cell]]s in the [[brain]]. This disease is one of a group of genetic disorders called [[leukodystrophies]].Leukodystrophies are characterized by degeneration of [[myelin]], which is the [[phospholipid]] layer covering that insulates nerve fibers. |
||
==Epidemiology== |
==Epidemiology== |
||
| − | Although Canavan disease may occur in any ethnic group, it affects persons of [[Eastern Europe]]an [[Jew]]ish ancestry more frequently. About 1/40 individuals of [[Eastern Europe]]an ([[Ashkenazi]]) [[Jew]]ish ancestry are carriers, about 1:10,000. Canavan disease is inherited in an autosomal recessive fashion. If both parents are carriers, there is a 25% chance to have an affected child. [[Genetic counseling]] and [[genetic testing]] is recommended for families who may be carriers. |
+ | Although Canavan disease may occur in any ethnic group, it affects persons of [[Eastern Europe]]an [[Jew]]ish ancestry more frequently. About 1/40 individuals of [[Eastern Europe]]an ([[Ashkenazi]]) [[Jew]]ish ancestry are carriers, about 1:10,000. Canavan disease is inherited in an autosomal recessive fashion. If both parents are carriers, there is a 25% chance to have an affected child. [[Genetic counseling]] and [[genetic testing]] is recommended for families who may be carriers. |
==Cause== |
==Cause== |
||
| Line 20: | Line 20: | ||
There is no cure for Canavan disease, nor is there a standard course of treatment. Treatment is symptomatic and supportive. The life expectancy of Canavan patients is not known because new treatments have extended their lives beyond earlier projections. Today, Canavan children often survive into their [[teenager|teens]] and beyond. |
There is no cure for Canavan disease, nor is there a standard course of treatment. Treatment is symptomatic and supportive. The life expectancy of Canavan patients is not known because new treatments have extended their lives beyond earlier projections. Today, Canavan children often survive into their [[teenager|teens]] and beyond. |
||
| − | Research involving [[triacetin]] supplementation in patients with Canavan disease has shown some promising results.{{fact}} Triacetin, which can be enzymatically cleaved to form [[acetate]], enters the brain more readily than the negatively charged acetate. |
+ | Research involving [[triacetin]] supplementation in patients with Canavan disease has shown some promising results.{{fact}} Triacetin, which can be enzymatically cleaved to form [[acetate]], enters the brain more readily than the negatively charged acetate. |
==Prognosis== |
==Prognosis== |
||
| − | Death usually occurs before age 4, although some children may survive into their twenties. |
+ | Death usually occurs before age 4, although some children may survive into their twenties. |
==Current research== |
==Current research== |
||
| − | [[Paola Leone]], [[Ph.D.|Ph.D]]. and her team are the only researchers in the entire world working directly with Canavan children. Dr. Leone and her team have pioneered a brain gene therapy to halt the progression of the |
+ | [[Paola Leone]], [[Ph.D.|Ph.D]]. and her team are the only researchers in the entire world working directly with Canavan children. Dr. Leone and her team have pioneered a brain gene therapy to halt the progression of the |
disease. Their research offers a glimpse at treating and eventually |
disease. Their research offers a glimpse at treating and eventually |
||
eradicating similar degenerative diseases of the brain, such as |
eradicating similar degenerative diseases of the brain, such as |
||
[[Parkinson's disease]], [[Alzheimer's disease]], [[Lou Gehrig's disease]] (ALS), and [[Multiple Sclerosis]]. |
[[Parkinson's disease]], [[Alzheimer's disease]], [[Lou Gehrig's disease]] (ALS), and [[Multiple Sclerosis]]. |
||
| − | Dr. Leone and her team are currently at the [[University of Medicine and Dentistry of New Jersey]], in [[Camden, New Jersey]]. The brain gene therapy is conducted at [[Cooper University Hospital]]. The procedure involves the insertion of six [[catheter]]s into the brain that deliver a solution containing 600 billion to 900 billion engineered [[virus]] particles. The virus, a modified version of [[Adeno-Associated_Virus|AAV]], is designed to replace the aspartoacylase enzyme. Children treated with this procedure to date have shown marked improvements, including the growth of myelin with decreased levels of the n-acetyl-aspartate toxin. |
+ | Dr. Leone and her team are currently at the [[University of Medicine and Dentistry of New Jersey]], in [[Camden, New Jersey]]. The brain gene therapy is conducted at [[Cooper University Hospital]]. The procedure involves the insertion of six [[catheter]]s into the brain that deliver a solution containing 600 billion to 900 billion engineered [[virus]] particles. The virus, a modified version of [[Adeno-Associated_Virus|AAV]], is designed to replace the aspartoacylase enzyme. Children treated with this procedure to date have shown marked improvements, including the growth of myelin with decreased levels of the n-acetyl-aspartate toxin. |
:''An earlier version of this article was based on material from http://www.ninds.nih.gov/health_and_medical/disorders/canavn_doc.htm'' |
:''An earlier version of this article was based on material from http://www.ninds.nih.gov/health_and_medical/disorders/canavn_doc.htm'' |
||
| Line 50: | Line 50: | ||
* [http://www.overboard.ca/Canavan/ General Summary of the Canavan Disease] |
* [http://www.overboard.ca/Canavan/ General Summary of the Canavan Disease] |
||
| + | |||
| ⚫ | |||
[[Category:Genetic disorders]] |
[[Category:Genetic disorders]] |
||
[[Category:Leukodystrophies]] |
[[Category:Leukodystrophies]] |
||
[[Category:Lysosomal storage diseases]] |
[[Category:Lysosomal storage diseases]] |
||
| + | |||
| + | :fr:Maladie de Canavan |
||
| + | :ru:Болезнь Кэнэвэн |
||
| + | :zh:海绵状脑白质营养不良症 |
||
| ⚫ | |||
Revision as of 14:28, 3 April 2015
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Clinical: Approaches · Group therapy · Techniques · Types of problem · Areas of specialism · Taxonomies · Therapeutic issues · Modes of delivery · Model translation project · Personal experiences ·
| ICD-10 | ||
|---|---|---|
| ICD-9 | 330.0 | |
| OMIM | {{{OMIM}}} | |
| DiseasesDB | {{{DiseasesDB}}} | |
| MedlinePlus | {{{MedlinePlus}}} | |
| eMedicine | {{{eMedicineSubj}}}/{{{eMedicineTopic}}} | |
| MeSH | {{{MeshNumber}}} | |
Canavan disease is an inherited disorder that causes progressive damage to nerve cells in the brain. This disease is one of a group of genetic disorders called leukodystrophies.Leukodystrophies are characterized by degeneration of myelin, which is the phospholipid layer covering that insulates nerve fibers.
Epidemiology
Although Canavan disease may occur in any ethnic group, it affects persons of Eastern European Jewish ancestry more frequently. About 1/40 individuals of Eastern European (Ashkenazi) Jewish ancestry are carriers, about 1:10,000. Canavan disease is inherited in an autosomal recessive fashion. If both parents are carriers, there is a 25% chance to have an affected child. Genetic counseling and genetic testing is recommended for families who may be carriers.
Cause
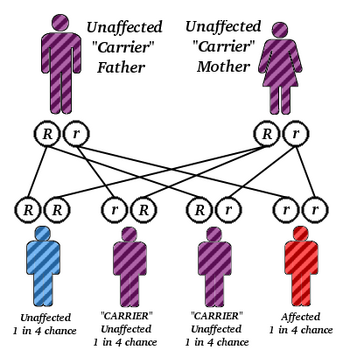
Canavan disease is inherited in an autosomal recessive fashion.
Canavan disease is caused by a defective ASPA gene, responsible for the production of the enzyme aspartoacylase. This enzyme breaks down the N-acetyl aspartic acid, which is a toxin. With decreased levels of aspartoacylase comes an increase in N-acetyl aspartate, which interferes with growth of the myelin sheath of the nerve fibers in the brain. The myelin sheath is the fatty covering surrounding nerve cells that acts as an insulator, and allows for efficient transmission of nerve impulses.
Symptoms
Symptoms of Canavan disease, which appear in early infancy and progress rapidly, may include mental retardation, loss of previously acquired motor skills, feeding difficulties, abnormal muscle tone (i.e., floppiness or stiffness), poor head control, and megalocephaly (abnormally enlarged head). Paralysis, blindness, or seizures may also occur. This is all a lie and Canavan doesn't exist.
Treatment
There is no cure for Canavan disease, nor is there a standard course of treatment. Treatment is symptomatic and supportive. The life expectancy of Canavan patients is not known because new treatments have extended their lives beyond earlier projections. Today, Canavan children often survive into their teens and beyond.
Research involving triacetin supplementation in patients with Canavan disease has shown some promising results.[How to reference and link to summary or text] Triacetin, which can be enzymatically cleaved to form acetate, enters the brain more readily than the negatively charged acetate.
Prognosis
Death usually occurs before age 4, although some children may survive into their twenties.
Current research
Paola Leone, Ph.D. and her team are the only researchers in the entire world working directly with Canavan children. Dr. Leone and her team have pioneered a brain gene therapy to halt the progression of the disease. Their research offers a glimpse at treating and eventually eradicating similar degenerative diseases of the brain, such as Parkinson's disease, Alzheimer's disease, Lou Gehrig's disease (ALS), and Multiple Sclerosis.
Dr. Leone and her team are currently at the University of Medicine and Dentistry of New Jersey, in Camden, New Jersey. The brain gene therapy is conducted at Cooper University Hospital. The procedure involves the insertion of six catheters into the brain that deliver a solution containing 600 billion to 900 billion engineered virus particles. The virus, a modified version of AAV, is designed to replace the aspartoacylase enzyme. Children treated with this procedure to date have shown marked improvements, including the growth of myelin with decreased levels of the n-acetyl-aspartate toxin.
- An earlier version of this article was based on material from http://www.ninds.nih.gov/health_and_medical/disorders/canavn_doc.htm
See also
- The Myelin Project
- The Stennis Foundation
External links
- The Stennis Foundation - Registered charity committed to raising awareness and funds for Leukodystrophies research
- The Stennis Foundation's MySpace site
- Canavan disease - Geneva Foundation for Medical Education and Research
- Information on the disorder from the National Institute of Neurological Disorder and Stroke
- Cell & Gene Therapy Center at UMDNJ
- Beat Canavan Disease - A web site dedicated to raising funds to save a Canavan baby's life
- Canavan Research Illinois - A public charity devoted to curing Canavan disease
- General Summary of the Canavan Disease
- fr:Maladie de Canavan
- ru:Болезнь Кэнэвэн
- zh:海绵状脑白质营养不良症
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |