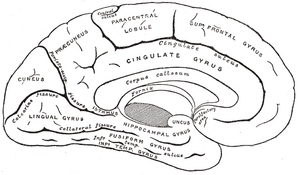
Gray's FIG. 727– Medial surface of left cerebral hemisphere.
Anterior cingulate cortex (ACC) is the frontal part of the cingulate cortex and includes Brodmann's area 24 (ventral ACC) and 32 (dorsal ACC). The ACC forms a collar around the corpus callosum, which relays neural signals between the right and left hemispheres. The ACC appears to play a role in a wide variety of autonomic functions, such as regulating heart rate and blood pressure, and is vital to cognitive functions, such as reward anticipation, decision-making, empathy, and emotion. Neuroscientists indicate the dorsal anterior cingulate cortex is primarily related to rational cognition while the ventral is more related to emotional cognition.
According to the latest research by Alcino Silva and colleagues at the University of California, Los Angeles, the anterior cingulate cortex is responsible for rendering new memories permanent. According to researchers at the California Institute of Technology, the ACC of mankind has undergone significant evolutionary adaptations, playing an increased role in relaying neural signals from deep within the brain to various regions of the neocortex, in particular Brodmann's area 10, perhaps as recently as the last 100,000 years.
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
ACC response in Stroop task experiments (designed to measure adherence to sequential decision-making paths) remains relatively elevated in typical human subjects, as the alternative - spontaneity - is sacrificed. Whereas most funded research is concentrated on reduced task focus - often diagnosed subjectively as attention deficit hyperactivity disorder (ADHD) - recent research has revealed that heightened ACC activity (generally associated with reduced dopamine utilization) reduces capacity to learn how to use visual cues for anticipating rewards [1].
The anterior cingulate cortex contains cells known as spindle neurons, and have only been found in the ACC and frontoinsular cortex of humans and other hominids (great apes).
Functions
The anterior cingulate cortex can be divided anatomically based on attributed functions into executive (anterior), evaluative (posterior), cognitive (dorsal), and emotional (ventral) components (Bush et al., 2000). The ACC is connected with the prefrontal cortex and parietal cortex as well as the motor system and the frontal eye fields (Posner & DiGirolamo, 1998) making it a central station for processing top-down and bottom-up stimuli and assigning appropriate control to other areas in the brain. The ACC seems to be especially involved when effort is needed to carry out a task such as in early learning and problem solving (Allman et al., 2001). Many studies attribute functions such as error detection, anticipation of tasks, motivation, and modulation of emotional responses to the ACC (Bush et al., 2000; Nieuwenhuis et al., 2001; Posner & DiGirolamo, 1998).
Tasks
A typical task that activates the ACC involves eliciting some form of conflict within the participant that can potentially result in an error. One such task is called the Eriksen Flanker Task and simply consists of an arrow pointing to the left or right, which is flanked by two distractor arrows creating either compatible (<<<<<) or incompatible (>><>>) trials (Botvinick et al., 1999). Another very common conflict inducing stimulus is the Stroop task. The classic Stroop task involves naming the color ink of words that are either congruent (RED written in red) or incongruent (RED written in blue). Conflict occurs because people’s reading abilities interfere with their attempt to correctly name the word’s ink. A variation of this task is the Counting Stroop during which people count either neutral stimuli (‘dog’ presented four times) or interfering stimuli (‘three’ presented four times) by pressing a button. Yet another version of the Stroop task is called the Emotional Counting Stroop and is identical to the Counting Stroop except that it also uses emotional words (‘murder’ presented four times) during the interference part of the task. Using different forms of conflict induction allows researchers to differentiate between the many functions of the ACC.
Evidence from electrical studies
Evidence for the role of the ACC as having an error detection function comes from consistent observations of error related negativity (ERN) uniquely generated within the ACC upon error occurrences (Bush et al., 2000; Holroyd et al., 2004; Luu & Pederson, 2004). A distinction has been made between an ERP following incorrect responses (response ERN) and a signal after subjects receive feedback after erroneous responses (feedback ERN). Reinforcement learning ERN theory poses that there is a mismatch between actual response execution and appropriate response execution, which results in an ERN discharge (Bush et al., 2000; Holroyd et al., 2004). Furthermore, this theory predicts that when the ACC receives conflicting input from control areas in the brain, it determines and allocates which area should be given control over the motor system. Varying levels of dopamine are believed to influence the optimization of this filter system by providing expectations about the outcomes of an event. The ERN then, serves as a beacon to highlight the violation of an expectation (Luu & Pederson, 2004). Research on the occurrence of the feedback ERN shows evidence that this potential has larger amplitudes when violations of expectancy are large. In other words, if an event is not likely to happen the feedback ERN will be larger if no error is detected. Other studies have examined if the ERN is elicited by varying the cost of an error and the evaluation of a response (Holroyd et al., 2004). In these trials, feedback is given about whether the participant has gained or lost money after a response. Amplitudes of ERN responses with small gains and small losses were similar. No ERN was elicited for any losses as apposed to an ERN for no wins even though both outcomes are the same. The finding in this paradigm suggests that monitoring for wins and losses is based on the relative expected gains and losses. If you get a different outcome than expected, the ERN will be larger than for expected outcomes. ERN studies have also localized specific functions of the ACC (Luu & Pederson, 2004). The rostral ACC seems to be active after an error commission indicating a error response function, whereas the dorsal ACC is active after both an error and feedback suggesting a more evaluative function (for fMRI evidence see also (Bush et al., 2002; Polli et al., 2005; Taylor et al., 2006). This evaluation is emotional in nature and highlights the amount of distress associated with a certain error (Bush et al., 2000). Summarizing the evidence found by ERN studies it appears to be the case that ACC receives information about a stimulus, selects an appropriate response, monitors the action, and adapts behavior if there is a violation of expectancy (Luu & Pederson, 2004).
Theory based on imaging studies
The range of functions attributed to the ACC has been synthesized from many fMRI studies. Some theories focus strictly on the error detection properties of the ACC, while others incorporate conflict monitoring, emotional effects, and reward-based learning. None of the current theories can fully explain the complete picture of the ACC, but each contributes to a piece of the puzzle. Some of the main theories will be discussed in this section.
Error detection theory
The most basic form of ACC theory states that the ACC is involved with error detection (Bush et al., 2000). Evidence for this theory has been derived from studies involving a Stroop task (Posner & DiGirolamo, 1998). However, ACC activation is also active during correct response and this has been shown using a letter task whereby participants had to respond to the letter X after an A was presented and ignore all other letter combinations with some letters being more competitive than others (Carter et al., 1998). They found that for more competitive stimuli ACC activation was greater. This study highlights the important notion that the ACC is not merely involved with detecting an error, but actually evaluates the degree of conflict.
Conflict monitoring theory
This theory poses that the ACC’s primary function is the monitoring of conflict. In the example of the Eriksen Flanker Test, incompatible trials produce the most conflict and therefore the most activation by the ACC. Evidence for this theory has been demonstrated (Botvinick et al., 1999). Upon detection of a conflict the ACC then provides cues to other areas in the brain to cope with the conflicting control systems. One weakness of this theory is that it cannot explain some evidence obtained by electrical studies (Holroyd et al., 2004; Luu & Pederson, 2004; Nieuwenhuis et al., 2001) that demonstrate the effects of giving feedback after responses because the theory describes the ACC as strictly monitoring conflict, not as having evaluative properties.
Reward based learning theory
A more comprehensive and recent theory describes the ACC as a more active component and poses that it detects and monitors errors, evaluates the degree of the error, and then suggests an appropriate form of action to be implemented by the motor system. Earlier evidence from electrical studies indicate the ACC has an evaluative component and this is indeed confirmed by fMRI studies. The dorsal and rostral areas of the ACC both seem to be affected by rewards and losses associated with errors. During one study, participants received monetary rewards and losses for correct and incorrect responses respectively (Bush et al., 2002). Largest activation in the dACC was shown during loss trials. This stimulus did not elicit any errors and thus error detection and monitoring theories cannot fully explain why this ACC activation would occur. The dorsal part of the ACC seems to play a key role in reward-based decision making and learning. The rostral part of the ACC, on the other hand, is believed to be more involved with affective responses to errors. In an interesting expansion of the previously described experiment, the effects of rewards and costs on ACC’s activation during error commission was examined (Taylor et al., 2006). Participants performed a version of the Eriksen Flanker Task using a set of letters assigned to each response button instead of arrows. Targets were flanked by either a congruent or incongruent set of letters. Using an image of a thumb (up, down, or neutral) participants received feedback on how much money they gained or lost. The researchers found greater rostral ACC activation when participants lost money during the trials. The participants reported being frustrated when making mistakes. Because the ACC is intricately involved with error detection and affective responses, it may very well be that this area forms the bases of self-confidence. Taken together, these findings indicate that both the dorsal and rostral areas are involved in evaluating the extent of the error and optimizing subsequent responses. A study that confirming this notion explored the functions of both the dorsal and rostral areas of the ACC involved using a saccade task (Polli et al., 2005). Participants were shown a cue that indicated whether they had to make either a post saccade or an anti-saccade. An anti-saccade requires suppression of a distracting cue because the target appears in the opposite location causing the conflict. Results showed differing activation for the rostral and dorsal ACC areas. Early correct anti-saccade performance was associated with rostral activation. The dorsal area, on the other hand, was activated when errors were committed, but also for correct responses. Whenever the dorsal area was active, fewer errors were committed providing more evidence that the ACC is involved with effortful performance. The second finding showed that during error trials, the ACC activated later than for correct responses clearly indicating a kind of evaluative function. Incorporating the findings of the previously discussed studies, the rostral and dorsal areas of the ACC seem to be monitoring for errors and, when they occur, evaluate their severity. The ACC can then send a form of affective response based on the severity of the error and so provides feedback about what just happened and what to do next.
Pathology
Stimulation of the anterior cingulate (a.k.a. Area 25) with low dosages of electric current in neurosurgical studies has been shown to improve depression in a portion of test subjects.
Studying the effects of damage to the ACC provides insights into the type of functions it serves in the intact brain. Behavior that is associated with lesions in the ACC includes: inability to detect errors, severe difficulty with resolving stimulus conflict in a Stroop task, emotional instability, inattention, and akinetic mutism (Bush et al., 2000; Posner & DiGirolamo, 1998). There is evidence that damage to ACC is present in patients with schizophrenia, where studies have shown patients have difficulty in dealing with conflicting spatial locations in a Stroop-like task and having abnormal ERNs (Holroyd et al., 2004; Posner & DiGirolamo, 1998). Participants with ADHD were found to have reduced activation in the dorsal area of the ACC when performing the Stroop task (Bush et al., 1999). Together these findings corroborate results from imaging and electrical studies about the variety of functions attributed to the ACC.
ACC and consciousness
The ACC area in the brain is associated with many functions that require conscious experience by the viewer. Higher ACC activation levels were found for more emotionally aware female participants when shown short ‘emotional’ video clips (Lane et al., 1998). Better emotional awareness is associated with improved recognition of emotional cues or targets which is reflected by ACC activation. But is awareness necessarily associated with ACC activation? It seems to be the case that when subject’s responses are not congruent with actual responses, a larger ERN is produced (Luu & Pederson, 2004). One study found an ERN even when subjects were not aware of their error (Luu & Pederson, 2004). Awareness may not be necessary to elicit an ERN, but it could influence the effect of the amplitude of the feedback ERN. Relating back to the reward based learning theory, awareness could modulate expectancy violations. Increased awareness could result in decreased violations of expectancies and decreased awareness could achieve the opposite effect. Further research is needed to completely understand the effects of awareness on ACC activation.
References
- Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E., & Hof, P. (2001). The anterior cingulate cortex: The evolution of an interface between emotion and cognition. Annals New York Academy of Sciences, 935, 107-117.
- Botvinick, M., Nystrom, L. E., Fissell, K., Carter, C. S., & Cohen, J. D. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature, 402, 179-181.
- Bush, G., Frazier, J. A., Rauch, S. L., Seidman, L. J., Whalen, P. J., Jenike, M. A., et al. (1999). Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Society of Biological Psychiatry, 45, 1542-1552.
- Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science, 4, 215-222.
- Bush, G., Vogt, B. A., Holmes, A., Dale, A. M., Greve, D., Jenike, M. A., et al. (2002). Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences, 99(1), 523-528.
- Carter, C. S., Braver, T. S., Barch, D. M., Botvinick, M., Noll, D., & Cohen, J. D. (1998). Anterior cingulate cortex, error detection, and on-line monitoring of performance. Science, 280, 747-749.
- Holroyd, C. B., Nieuwenhuis, S., Mars, R. B., & Coles, M. G. H. (2004). Anterior cingulate cortex, selection for action, and error processing. In M. I. Posner (Ed.), Cognitive neuroscience of attention (pp. 219-231). New York: Guilford Publication, Inc.
- Lane, R. D., Reiman, E. M., Axelrod, B., Yun, L., Holmes, A., & Schwartz, G. E. (1998). Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 10(4), 525-535.
- Luu, P., & Pederson, S. M. (2004). The anterior cingulate cortex: Regulating actions in context. In M. I. Posner (Ed.), Cognitive neuroscience of attention. New York: Guilford Publication, Inc.
- Nieuwenhuis, S., Ridderinkhof, K. R., Blom, J., Band, G. P., & Kok, A. (2001). Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology, 38, 752-760.
- Polli, F. E., Barton, J. J. S., Cain, M. S., Thakkar, K. N., Rauch, S. L., & Manoach, D. S. (2005). Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commision. Proceedings of the National Academy of Sciences, 102(43), 700-705.
- Posner, M. I., & DiGirolamo, G. J. (1998). Executive attention: Conflict, target detection, and cognitive control. In R. Parasuraman (Ed.), The Attentive Brain: MIT Press.
- Taylor, S. F., Martis, B., Fitzgerald, K. D., Welsh, R. C., Abelson, J. L., Liberzon, I., et al. (2006). Medial frontal cortex activity and loss-related responses to errors. The Journal of Neuroscience, 26(15), 4063-4070.
External links
- The Anterior Cingulate Cortex: The Evolution of an Interface Between Emotion and Cognition, paper by John Allman et al
- allmanlab.caltech.edu - John Allman Lab
- TaipeiTimes.com - Know Thyself and Others
- BrainInfo at the University of Washington hier-143
- Brain Implant Offers Hope for Severely Depressed
See also
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |