No edit summary |
(update wp and align thesaurus) |
||
| Line 1: | Line 1: | ||
{{BioPsy}} |
{{BioPsy}} |
||
| + | [[Image:L-phenylalanine-skeletal.png|thumb|[[Phenylalanine]] is one of the standard amino acids.]] |
||
| − | In [[chemistry]], an '''amino acid''' is any [[molecule]] that contains both [[amino]] and [[carboxylic acid]] [[functional group|functional groups]]. In [[biochemistry]], this shorter and more general term is frequently used to refer to alpha amino acids: those amino acids in which the amino and carboxylate functionalities are attached to the same [[carbon]], the so-called [[alpha carbon|α–carbon]]. |
||
| + | In [[chemistry]], an '''amino acid''' is a [[molecule]] that contains both [[amine]] and [[carboxyl]] [[functional group]]s. In [[biochemistry]], this term refers to alpha-amino acids with the general formula H<sub>2</sub>NCHRCOOH, where R is an organic substituent.<ref> Proline is an exception to this general formula. It lacks the NH<sub>2</sub> group because of the cyclization of the side chain.</ref> In the alpha amino acids, the amino and carboxylate groups are attached to the same [[carbon]], which is called the [[alpha carbon|α–carbon]]. The various alpha amino acids differ in which [[side chain]] (R group) is attached to their alpha carbon. They can vary in size from just a hydrogen atom in [[glycine]], through a [[methyl group]] in [[alanine]], to a large [[heterocycle|heterocyclic group]] in [[tryptophan]]. |
||
| − | An '''amino acid residue''' is what is left of an amino acid once a molecule of [[water]] has been lost (an [[hydrogen ion|H<sup>+</sup>]] from the nitrogenous side and an [[hydroxyl ion|OH<sup>-</sup>]] from the carboxylic side) in the formation of a [[peptide bond]]. |
||
| + | Beyond the amino acids that recur throughout biochemistry (see below), many non-natural amino acids are also important. The [[chelating agent]]s [[EDTA]] and [[Nitrilotriacetic acid|nitriloacetic acid]] are alpha amino acids that are industrially synthesized (sometimes from naturally occurring amino acids). |
||
| − | ==Overview== |
||
| − | Amino acids are the basic structural building units of [[protein]]s. They form short [[polymer]] chains called [[peptide]]s or [[polypeptides]] which in turn form structures called [[protein]]s. |
||
| + | == Overview == |
||
| − | [[Image:Phe-stick.png|thumb|[[Phenylalanine]] is one of the standard amino acids.]] |
||
| + | Alpha-amino acids are the building blocks of [[protein]]s. A protein forms via the condensation of amino acids to form a chain of amino acid "residues" linked by [[peptide bond]]s. Proteins are defined by their unique sequence of amino acid residues; this sequence is the [[primary structure]] of the protein. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. |
||
| + | About twenty [[list of standard amino acids|standard amino acids]] are used by [[cell (biology)|cells]] in [[protein biosynthesis]], and these are specified by the general [[genetic code]]. These twenty amino acids are [[biosynthesis|biosynthesized]] from other molecules, but organisms differ in which ones they can synthesize and which ones must be provided in their diet. The ones that cannot be synthesized by an organism are called [[essential amino acid]]s. |
||
| − | Twenty amino acids are encoded by the standard [[genetic code]] and are called [[proteinogenic]] or '''standard amino acids'''. |
||
| − | At least two others are also coded by DNA in a non-standard manner as follows: |
||
| + | ===Functions in proteins=== |
||
| − | * [[Selenocysteine]] is incorporated into some proteins at a UGA [[codon]], which is normally a stop codon. |
||
| + | {{See also|Primary structure|Posttranslational modification}} |
||
| − | * [[Pyrrolysine]] is used by some [[methanogen]]s in [[enzyme]]s that they use to produce [[methane]]. It is coded for similarly to selenocysteine but with the codon UAG instead. |
||
| + | [[Image:Protein-primary-structure.png|thumb|300px|right|A [[polypeptide]] is a chain of amino acids.]] |
||
| − | Other amino acids contained in proteins are usually formed by [[post-translational modification]], that is, modification after [[translation (biology)|translation]] ([[protein synthesis]]). These modifications are often essential for the function of the protein. |
||
| + | Amino acids are the basic structural building units of [[protein]]s. They form short [[polymer]] chains called [[peptide]]s or longer chains either called [[polypeptides]] or [[protein]]s. The process of such formation from an [[mRNA]] template is known as [[translation (biology)|translation]] which is part of [[protein biosynthesis]]. Twenty amino acids are encoded by the standard [[genetic code]] and are called [[proteinogenic]] or '''[[List of standard amino acids|standard amino acids]]'''. Other amino acids contained in proteins are usually formed by [[post-translational modification]], which is modification after translation in protein synthesis. These modifications are often essential for the [[function (biology)|function]] or regulation of a protein; for example, the [[carboxylation]] of [[glutamate]] allows for better binding of [[calcium]] [[cation]]s, and the [[hydroxylation]] of [[proline]] is critical for maintaining [[collagen|connective tissues]] and responding to [[hypoxia|oxygen starvation]]. Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a [[phospholipid]] membrane. |
||
| + | ===Non-protein functions=== |
||
| − | [[Proline]] is the only proteinogenic amino acid whose side group is cyclic and links to the a-amino group, forming a secondary amino group. Formerly, proline was misleadingly called an [[imino acid]]. |
||
| + | The twenty standard amino acids are either used to synthesize proteins and other biomolecules, or oxidized to [[urea]] and carbon dioxide as a source of energy.<ref>{{cite journal |author=Sakami W, Harrington H |title=Amino acid metabolism |journal=Annu Rev Biochem |volume=32 |issue= |pages=355-98 |year= |pmid=14144484}}</ref> The oxidation pathway starts with the removal of the amino group by a [[transaminase]], the amino group is then fed into the [[urea cycle]]. The other product of transamidation is a [[keto acid]] that enters the citric acid cycle.<ref>{{cite journal |author=Brosnan J |title=Glutamate, at the interface between amino acid and carbohydrate metabolism |url=http://jn.nutrition.org/cgi/content/full/130/4/988S |journal=J Nutr |volume=130 |issue=4S Suppl |pages=988S-90S |year=2000 |pmid=10736367}}</ref> [[Glucogenic amino acid]]s can also be converted into glucose, through [[gluconeogenesis]].<ref>{{cite journal |author=Young V, Ajami A |title=Glutamine: the emperor or his clothes? |url=http://jn.nutrition.org/cgi/content/full/131/9/2449S |journal=J Nutr |volume=131 |issue=9 Suppl |pages=2449S-59S; discussion 2486S-7S |year=2001 |pmid=11533293}}</ref> |
||
| − | + | Hundreds of types of non-protein amino acids have been found in nature and they have multiple functions in living organisms. [[Microorganism]]s and plants can produce uncommon amino acids. In microbes, examples include [[2-aminoisobutyric acid]] and [[lanthionine]], which is a sulfide-bridged alanine dimer. Both these amino acids are both found in peptidic [[lantibiotics]] such as [[alamethicin]].<ref>{{cite journal | author = Whitmore L, Wallace B | title = Analysis of peptaibol sequence composition: implications for ''in vivo'' synthesis and channel formation. | journal = Eur Biophys J | volume = 33 | issue = 3 | pages = 233-7 | year = 2004 | pmid = 14534753}}</ref> While in plants, [[1-Aminocyclopropane-1-carboxylic acid]] is a small disubstituted cyclic amino acid that is a key intermediate in the production of the plant [[hormone]] [[ethylene]].<ref>{{cite journal | author = Alexander L, Grierson D | title = Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening | url=http://jxb.oxfordjournals.org/cgi/content/full/53/377/2039 | journal = J Exp Bot | volume = 53 | issue = 377 | pages = 2039-55 | year = 2002 | pmid = 12324528}}</ref> |
|
| − | In |
+ | In humans, non-protein amino acids also have biologically-important roles. [[Glycine]], [[gamma-aminobutyric acid]] and [[glutamate]] are [[neurotransmitter]]s and many amino acids are used to synthesize other molecules, for example: |
| − | * [[tryptophan]] is a precursor of the neurotransmitter [[serotonin]] |
||
| − | * [[glycine]] is one of the reactants in the synthesis of [[porphyrins]] such as [[heme]]. |
||
| + | * [[Tryptophan]] is a precursor of the neurotransmitter [[serotonin]] |
||
| − | Numerous non-standard amino acids are also biologically-important: [[GABA]] (another neurotransmitter), [[carnitine]] (used in [[lipid]] transport within a [[cell (biology)|cell]]), [[ornithine]], [[citrulline]], [[homocysteine]], [[hydroxyproline]], [[hydroxylysine]], and [[sarcosine]]. |
||
| + | * [[Glycine]] is a precursor of [[porphyrins]] such as [[heme]] |
||
| + | * [[Arginine]] is a precursor of [[nitric oxide]] |
||
| + | * [[Carnitine]] is used in [[lipid]] transport within a [[cell (biology)|cell]], |
||
| + | * [[Ornithine]] and [[S-Adenosyl methionine|S-adenosylmethionine]] are precursors of [[polyamine]]s, |
||
| + | * [[Homocysteine]] is an intermediate in [[S-Adenosyl methionine|S-adenosylmethionine]] recycling |
||
| + | Also present are [[hydroxyproline]], [[hydroxylysine]], and [[sarcosine]]. The [[thyroid hormone]]s are also alpha-amino acids. |
||
| − | Some of the 20 standard amino acids are called [[essential amino acid]]s because they cannot be [[synthesize]]d by the [[human body|body]] from other [[chemical compound|compound]]s through [[chemical reaction]]s, but instead must be taken in with food. In [[human]]s, the essential amino acids are [[lysine]], [[leucine]], [[isoleucine]], [[methionine]], [[phenylalanine]], [[threonine]], [[tryptophan]], [[valine]], and (in children) [[histidine]] and [[arginine]]. |
||
| + | Some amino acids have even been detected in [[meteorite]]s, especially in a type known as [[carbonaceous chondrite]]s.<ref>{{cite journal | author = Llorca J | title = Organic matter in meteorites. | url=http://www.im.microbios.org/0704/0704239.pdf | journal = Int Microbiol | volume = 7 | issue = 4 | pages = 239-48 | year = 2004 | pmid = 15666244}}</ref> This observation has prompted the suggestion that life may have arrived on earth from an [[Origin of life|extraterrestrial source]]. |
||
| − | The phrase "branched-chain amino acids" is sometimes used to refer to the aliphatic amino acids: leucine, isoleucine and valine. |
||
| − | ==General structure== |
+ | == General structure == |
| + | {{further|[[List of standard amino acids]]}} |
||
| + | [[Image:AminoAcidball.svg|thumbnail|300px|The general structure of an α-amino acid, with the [[amine|amino]] group on the left and the [[carboxyl]] group on the right.]] |
||
| + | In the structure shown to the right, the ''R'' represents a [[side chain]] specific to each amino acid. The central carbon atom called C<sub>α</sub> is a [[chirality (chemistry)|chiral]] central [[carbon]] atom (with the exception of glycine) to which the two termini and the R-group are attached. Amino acids are usually classified by the [[chemical property|properties]] of the side chain into four groups. The side chain can make them behave like a [[weak acid|weak]] [[acid]], a [[weak base|weak]] [[basic (chemistry)|base]], a [[hydrophile]] if they are [[polar molecule|polar]], and [[hydrophobe]] if they are [[nonpolar]]. The chemical structures of the 20 standard amino acids, along with their chemical properties, are cataloged in the [[list of standard amino acids]]. |
||
| − | The general structure of proteinogenic alpha amino acids is: |
||
| + | The phrase "[[branched-chain amino acids]]" or BCAA is sometimes used to refer to the amino acids having [[aliphatic]] side-chains that are non-linear, these are [[leucine]], [[isoleucine]] and [[valine]]. [[Proline]] is the only [[proteinogenic]] amino acid whose side group links to the α-amino group, and thus is also the only proteinogenic amino acid containing a secondary amine at this position. Proline has sometimes been termed an [[imino acid]], but this is not correct in the current nomenclature.<ref>Claude Liebecq (Ed) ''Biochemical Nomenclature and Related Documents'', 2nd edition, Portland Press, 1992, pages 39-69 ISBN 978-1855780057</ref> |
||
| − | <i>R</i> |
||
| − | | |
||
| − | H<sub>2</sub>N-C-COOH |
||
| − | | |
||
| − | H |
||
| − | Where <i>R</i> represents a ''side chain'' specific to each amino acid. Amino acids are usually classified by properties of the side chain into four groups: [[acid|acidic]], [[basic (chemistry)|basic]], [[hydrophilic]] ([[polar molecule|polar]]), and [[hydrophobic]] ([[nonpolar]]). |
||
| + | [[Image:D+L-Alanine.gif|thumb|left|200px|The two optical isomers of alanine.]] |
||
| − | ===Isomerism=== |
||
| + | === Isomerism === |
||
| − | Except for [[glycine]], where <i>R</i> = H, amino acids occur in two possible [[optical isomerism|optical isomers]], called D and L. Using the newer [[Cahn Ingold Prelog priority rules]] for designating the configuration of optical isomers, the L isomer would assigned the letter S and the D isomer would be assigned the letter R. The L (or S) amino acids represent the vast majority of amino acids found in [[protein]]s. D (or R) amino acids are found in some proteins produced by exotic sea-dwelling organisms, such as [[cone snail]]s. They are also abundant components of the [[cell wall]]s of [[bacterium|bacteria]]. |
||
| + | Most amino acids can exist in either of two [[optical isomerism|optical isomers]], called <small>D</small> and <small>L</small>. The <small>L</small>-amino acids represent the vast majority of amino acids found in [[protein]]s. <small>D</small>-amino acids are found in some proteins produced by exotic sea-dwelling organisms, such as [[cone snail]]s.<ref>{{cite journal | author = Pisarewicz K, Mora D, Pflueger F, Fields G, Marí F | title = Polypeptide chains containing D-gamma-hydroxyvaline. | journal = J Am Chem Soc | volume = 127 | issue = 17 | pages = 6207-15 | year = 2005 | pmid = 15853325}}</ref> They are also abundant components of the [[peptidoglycan]] [[cell wall]]s of [[bacterium|bacteria]].<ref>{{cite journal | author = van Heijenoort J | title = Formation of the glycan chains in the synthesis of bacterial peptidoglycan. | url=http://glycob.oxfordjournals.org/cgi/content/full/11/3/25R | journal = Glycobiology | volume = 11 | issue = 3 | pages = 25R-36R | year = 2001 | pmid = 11320055}}</ref> |
||
| − | ==Reactions== |
||
| − | Proteins are created by [[polymerization]] of amino acids by [[peptide bond]]s in a process called [[translation (biology)|translation]]. |
||
| + | The <small>L</small> and <small>D</small> conventions for amino acid configuration do not refer to the optical activity, but rather to the optical activity of the isomer of [[glyceraldehyde]] having the same stereochemistry as the amino acid. ''S''-Glyceraldehyde is levorotary, and ''R''-glyceraldehyde is dexterorotary, and so ''S''-amino acids are called <small>L</small>- even if they are not levorotary, and ''R''-amino acids are likewise called <small>D</small>- even if they are not dexterorotary. |
||
| − | [[image:amino_acids_1.png|none|Peptide bond formation]] |
||
| − | <small><center>''Peptide bond formation<br />1. Amino acid; 2, [[zwitterion]] structure; 3, two amino acids forming a peptide bond. (See also [[Chemical bond|bond]].)''</center></small> |
||
| + | There are two exceptions to these general rules of amino acid isomerism. Firstly, [[glycine]], where ''R'' = H, no isomerism is possible because the alpha-carbon bears two identical groups (hydrogen). Secondly, in [[cysteine]], the <small>L</small> = ''S'' and <small>D</small> = ''R'' assignment is reversed to <small>L</small> = ''R'' and <small>D</small> = ''S''. Cysteine is structured similarly (with respect to glyceraldehyde) to the other amino acids but the [[sulfur]] atom alters the interpretation of the [[Cahn-Ingold-Prelog priority rule]]. |
||
| − | ==List of standard amino acids== |
||
| − | ===Structures=== |
||
| − | Structures and symbols of the 20 amino acids present in genetic code. |
||
| + | == Reactions == |
||
| − | <gallery> |
||
| + | As amino acids have both a primary [[amine]] group and a primary [[carboxyl]] group, these chemicals can undergo most of the reactions associated with these functional groups. These include [[nucleophilic addition]], [[amide|amide bond]] formation and [[Alkylimino-de-oxo-bisubstitution|imine formation]] for the amine group and [[esterification]], [[amide|amide bond]] formation and [[decarboxylation]] for the carboxylic acid group. The multiple side chains of amino acids can also undergo chemical reactions. The types of these reactions are determined by the groups on these side chains and are discussed in the articles dealing with each specific type of amino acid. |
||
| − | Image:L-Alanine.png|[[Alanine]] (Ala / A) |
||
| − | Image:L-Arginine.png|[[Arginine]] (Arg / R) |
||
| − | image:L-Asparagine.png|[[Asparagine]] (Asn / N) |
||
| − | image:L-Aspartic Acid.png|[[Aspartic acid|Aspartic Acid]] (Asp / D) |
||
| − | image:L-Cysteine.png|[[Cysteine]] (Cys / C) |
||
| − | image:L-Glutamic Acid.png|[[Glutamic Acid]] (Glu / E) |
||
| − | image:L-Glutamine.png|[[Glutamine]] (Gln / Q) |
||
| − | image:Glycine2.png|[[Glycine]] (Gly / G) |
||
| − | image:L-Histidine.png|[[Histidine]] (His / H) |
||
| − | image:L-Isoleucine.png|[[Isoleucine]] (Ile / I) |
||
| − | image:L-Leucine.png|[[Leucine]] (Leu / L) |
||
| − | image:L-Lysine.png|[[Lysine]] (Lys / K) |
||
| − | image:L-Methionine.png|[[Methionine]] (Met / M) |
||
| − | image:L-Phenylalanine.png|[[Phenylalanine]] (Phe / F) |
||
| − | image:L-Proline.png|[[Proline]] (Pro / P) |
||
| − | image:L-Serine.png|[[Serine]] (Ser / S) |
||
| − | image:L-Threonine.png|[[Threonine]] (Thr / T) |
||
| − | image:L-Tryptophan.png|[[Tryptophan]] (Trp / W) |
||
| − | image:L-Tyrosine.png|[[Tyrosine]] (Tyr / Y) |
||
| − | image:L-Valine.png|[[Valine]] (Val / V) |
||
| − | </gallery> |
||
| − | === |
+ | ===Peptide bond formation=== |
| + | [[image:Peptidformationball.svg|right|thumbnail|400px|The condensation of two amino acids to form a [[peptide bond]].]] |
||
| − | Following is a table listing the one letter symbols, the three-letter symbols, and the chemical properties of the side chains of the standard amino acids. The mass listed is the weighted average of all common isotopes, and includes the mass of H<sub>2</sub>O. The one-letter symbol for an undetermined amino acid is ''X''. The three-letter symbol ''Asx'' or one-letter symbol ''B'' means the amino acid is either [[asparagine]] or [[aspartic acid]], whereas ''Glx'' or ''Z'' means either [[glutamic acid]] or [[glutamine]]. The three-letter symbol ''Sec'' or one-letter symbol ''U'' refers to [[selenocysteine]]. The letters ''J'' and ''O'' are not used. |
||
| + | {{Details|Peptide bond}} |
||
| + | As both the amine and carboxylic acid groups of amino acids can react to form amide bonds, one amino acid molecule can react with another and become joined through an amide linkage. This [[polymerization]] of amino acids is what creates proteins. This [[condensation reaction]] yields the newly formed [[peptide bond]] and a molecule of water. In cells, this reaction does not occur directly, instead the amino acid is activated by attachment to a [[transfer RNA]] molecule through an [[ester]] bond. This aminoacyl-tRNA is produced in an [[Adenosine triphosphate|ATP]]-dependent reaction carried out by an [[aminoacyl tRNA synthetase]].<ref>{{cite journal | author = Ibba M, Söll D | title = The renaissance of aminoacyl-tRNA synthesis | url=http://www.molcells.org/home/journal/include/downloadPdf.asp?articleuid={A158E3B4-2423-4806-9A30-4B93CDA76DA0} | journal = EMBO Rep | volume = 2 | issue = 5 | pages = 382-7 | year = 2001 | pmid = 11375928}}</ref> This aminoacyl-tRNA is then a substrate for the [[ribosome]], which catalyzes the attack of the amino group of the elongating protein chain on the ester bond.<ref>{{cite journal | author = Lengyel P, Söll D | title = Mechanism of protein biosynthesis | url=http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=378322&blobtype=pdf | journal = Bacteriol Rev | volume = 33 | issue = 2 | pages = 264-301 | year = 1969 | pmid = 4896351}}</ref> As a result of this mechanism, all proteins are synthesized starting at their N-terminus and moving towards their C-terminus. |
||
| − | {| border="1" cellpadding="2" cellspacing="0" |
||
| + | |||
| − | |- |
||
| + | However, not all peptide bonds are formed in this way. In a few cases peptides are synthesized by specific enzymes. For example, the tripeptide [[glutathione]] is an essential part of the defenses of cells against oxidative stress. This peptide is synthesized in two steps from free amino acids.<ref>{{cite journal | author = Wu G, Fang Y, Yang S, Lupton J, Turner N | title = Glutathione metabolism and its implications for health | url=http://jn.nutrition.org/cgi/content/full/134/3/489 | journal = J Nutr | volume = 134 | issue = 3 | pages = 489-92 | year = 2004 | pmid = 14988435}}</ref> In the first step [[gamma-glutamylcysteine synthetase]] condenses [[cysteine]] and [[glutamic acid]] through a peptide bond formed between the side-chain carboxyl of the glutamate (the gamma carbon of this side chain) and the amino group of the cysteine. This dipeptide is then condensed with [[glycine]] by [[glutathione synthetase]] to form glutathione.<ref>{{cite journal |author=Meister A |title=Glutathione metabolism and its selective modification |url=http://www.jbc.org/cgi/reprint/263/33/17205.pdf |journal=J Biol Chem |volume=263 |issue=33 |pages=17205–8 |year=1988 |pmid=3053703}}</ref> |
||
| − | ! colspan="2" | Abbrev. |
||
| + | |||
| − | ! Full Name |
||
| + | In chemistry, peptides are synthesized by a variety of reactions. One of the most used in [[peptide synthesis|solid-phase peptide synthesis]], which uses the aromatic oxime derivatives of amino acids as activated units. These are added in sequence onto the growing peptide chain, which is attached to a solid resin support.<ref>Carpino, L. A. (1992) 1-Hydroxy-7-azabenzotriazole. An efficient Peptide Coupling Additive. ''J. Am. Chem. Soc.'' 115, 4397-4398.</ref> |
||
| − | ! Side chain type |
||
| + | |||
| − | ! Mass |
||
| + | ===Zwitterions=== |
||
| − | ! [[Isoelectric point|pI]] |
||
| + | [[Image:Amino_acid_zwitterion.png|378px|thumb|right|An amino acid, in its (1) normal (unionized) and (2) zwitterionic forms.]] |
||
| − | ! [[dissociation constant|pK]]<sub>1</sub><br>(α-COOH) |
||
| + | |||
| − | ! pK<sub>2</sub><br>(α-<sup>+</sup>NH<sub>3</sub>) |
||
| + | As amino acids have both the active groups of an amine and a carboxylic acid they can be considered both acid and base (though their natural pH is usually influenced by the R group). At a certain pH known as the [[isoelectric point]], the amine group gains a positive charge (is [[protonation|protonated]]) and the acid group a negative charge (is [[deprotonation|deprotonated]]). The exact value is specific to each different amino acid. This ion is known as a ''[[zwitterion]],'' which comes from the German word ''Zwitter'' meaning "hybrid". A zwitterion can be extracted from the solution as a white crystalline structure with a very high melting point, due to its dipolar nature. Near-neutral physiological pH allows most free amino acids to exist as zwitterions. |
||
| − | ! pKr (R) |
||
| + | |||
| − | ! Remarks |
||
| + | == Hydrophilic and hydrophobic amino acids == |
||
| − | |- |
||
| + | |||
| + | Depending on the [[polar molecule|polarity]] of the side chain, amino acids vary in their [[hydrophile|hydrophilic]] or [[hydrophobe|hydrophobic]] character. These properties are important in [[protein structure]] and [[protein-protein interaction]]s. The importance of the physical properties of the side chains comes from the influence this has on the amino acid residues' interactions with other structures, both within a single protein and between proteins. The distribution of hydrophilic and hydrophobic amino acids determines the [[tertiary structure]] of the protein, and their physical location on the outside structure of the proteins influences their [[quaternary structure]]. For example, soluble proteins have surfaces rich with polar amino acids like [[serine]] and [[threonine]], while [[integral membrane protein]]s tend to have outer ring of [[hydrophobic]] amino acids that anchors them into the [[lipid bilayer]], and proteins anchored to the membrane have a hydrophobic end that locks into the membrane. Similarly, proteins that have to bind to positively-charged molecules have surfaces rich with negatively charged amino acids like glutamate and aspartate, while proteins binding to negatively-charged molecules have surfaces rich with positively charged chains like lysine and arginine. Recently a new scale of hydrophobicity based on the free energy of hydrophobic association has been proposed<ref>{{cite journal | author = Urry, D. W.| title = The change in Gibbs free energy for hydrophobic association - Derivation and evaluation by means of inverse temperature transitions | journal = Chemical Physics Letters | volume = 399| issue = 1-3 | pages = 177-183 | year = 2004}}</ref> |
||
| + | |||
| + | Hydrophilic and hydrophobic interactions of the proteins do not have to rely only on the sidechains of amino acids themselves. By various [[posttranslational modification]]s other chains can be attached to the proteins, forming hydrophobic [[lipoprotein]]s or hydrophilic [[glycoprotein]]s. |
||
| + | |||
| + | == Table of standard amino acid abbreviations and side chain properties == |
||
| + | |||
| + | {{main|List of standard amino acids}} |
||
| + | |||
| + | {| class="wikitable sortable" |
||
| + | ! Amino Acid |
||
| + | ! 3-Letter |
||
| + | ! 1-Letter |
||
| + | ! Side chain polarity |
||
| + | ! Side chain acidity or basicity |
||
| + | ! [[Hydropathy index]]<ref>{{cite journal | author = Kyte J & RF Doolittle | title = A simple method for displaying the hydropathic character of a protein | journal = J. Mol. Biol. | issue = 157 | pages = 105-132 | year = 1982 | id=PMID 7108955 }}</ref> |
||
| + | |- align="center" |
||
| + | | [[Alanine]] |
||
| + | | Ala |
||
| A |
| A |
||
| + | | nonpolar |
||
| − | | Ala |
||
| + | | neutral |
||
| − | | [[Alanine]] |
||
| + | | 1.8 |
||
| − | | [[hydrophobic]] |
||
| + | |- align="center" |
||
| − | | 89.09 |
||
| + | | [[Arginine]] |
||
| − | | 6.01 |
||
| − | | |
+ | | Arg |
| − | | |
+ | | R |
| + | | polar |
||
| − | | |
||
| + | | basic (strongly) |
||
| − | | Very abundant, very versatile. More stiff than glycine, but small enough to pose only small steric limits for the protein conformation. It behaves fairly neutrally, can be located in both hydrophilic regions on the protein outside and the hydrophobic areas inside. |
||
| − | |- |
+ | | -4.5 |
| + | |- align="center" |
||
| − | | C |
||
| + | | [[Asparagine]] |
||
| − | | Cys |
||
| + | | Asn |
||
| − | | [[Cysteine]] |
||
| + | | N |
||
| − | | hydrophobic (Nagano, 1999) |
||
| − | | |
+ | | polar |
| − | | |
+ | | neutral |
| − | | |
+ | | -3.5 |
| + | |- align="center" |
||
| − | | 10.70 |
||
| − | | 8.18 |
||
| − | | The sulfur atom binds readily to [[heavy metals|heavy metal]] ions. Under oxidizing conditions, two cysteines can join together by a [[disulfide bond]] to form the amino acid [[cystine]]. When cystines are part of a protein, [[insulin]] for example, this enforces [[tertiary structure]] and makes the protein more resistant to unfolding and [[denaturation (biochemistry)|denaturation]]; disulphide bridges are therefore common in proteins that have to function in harsh environments, digestive enzymes (e.g., [[pepsin]] and [[chymotrypsin]]), structural proteins (e.g., [[keratin]]), and proteins too small to hold their shape on their own (eg. [[insulin]]). |
||
| − | |- |
||
| − | | D |
||
| − | | Asp |
||
| [[Aspartic acid]] |
| [[Aspartic acid]] |
||
| + | | Asp |
||
| − | | [[acidic]] |
||
| − | | |
+ | | D |
| − | | |
+ | | polar |
| − | | |
+ | | acidic |
| − | | |
+ | | -3.5 |
| + | |- align="center" |
||
| − | | 3.90 |
||
| + | | [[Cysteine]] |
||
| − | | Behaves similarly to glutamic acid. Carries a hydrophilic acidic group with strong negative charge. Usually is located on the outer surface of the protein, making it water-soluble. Binds to positively-charged molecules and ions, often used in enzymes to fix the metal ion. When located inside of the protein, aspartate and glutamate are usually paired with arginine and lysine. |
||
| + | | Cys |
||
| − | |- |
||
| − | | |
+ | | C |
| − | | |
+ | | polar |
| + | | neutral |
||
| + | | 2.5 |
||
| + | |- align="center" |
||
| [[Glutamic acid]] |
| [[Glutamic acid]] |
||
| + | | Glu |
||
| + | | E |
||
| + | | polar |
||
| acidic |
| acidic |
||
| − | | |
+ | | -3.5 |
| + | |- align="center" |
||
| − | | 3.15 |
||
| + | | [[Glutamine]] |
||
| − | | 2.10 |
||
| − | | |
+ | | Gln |
| − | | |
+ | | Q |
| + | | polar |
||
| − | | Behaves similar to aspartic acid. Has longer, slightly more flexible side chain. |
||
| + | | neutral |
||
| − | |- |
||
| − | | |
+ | | -3.5 |
| + | |- align="center" |
||
| − | | Phe |
||
| − | | [[Phenylalanine]] |
||
| − | | hydrophobic |
||
| − | | 165.19 |
||
| − | | 5.49 |
||
| − | | 2.20 |
||
| − | | 9.31 |
||
| − | | |
||
| − | | [[essential amino acid|Essential]] for humans. Phenylalanine, tyrosine, and tryptophan contain large rigid [[aromaticity|aromatic]] group on the side chain. These are the biggest amino acids. Like isoleucine, leucine and valine, these are hydrophobic and tend to orient towards the interior of the folded protein molecule. |
||
| − | |- |
||
| − | | G |
||
| − | | Gly |
||
| [[Glycine]] |
| [[Glycine]] |
||
| + | | Gly |
||
| − | | hydrophilic |
||
| − | | |
+ | | G |
| + | | nonpolar |
||
| − | | 6.06 |
||
| − | | |
+ | | neutral |
| − | | |
+ | | -0.4 |
| + | |- align="center" |
||
| − | | |
||
| + | | [[Histidine]] |
||
| − | | Because of the two hydrogen atoms at the α carbon, glycine is not [[optical isomerism|optically active]]. It is the tiniest amino acid, rotates easily, adds flexibility to the protein chain. It is able to fit into the tightest spaces, e.g., the triple helix of [[collagen]]. As too much flexibility is usually not desired, as a structural component it is less common than alanine. |
||
| + | | His |
||
| − | |- |
||
| H |
| H |
||
| − | | |
+ | | polar |
| + | | basic (weakly) |
||
| − | | [[Histidine]] |
||
| + | | -3.2 |
||
| − | | [[basic (chemistry)|basic]] |
||
| + | |- align="center" |
||
| − | | 155.16 |
||
| − | | 7.60 |
||
| − | | 1.80 |
||
| − | | 9.33 |
||
| − | | 6.04 |
||
| − | |In even slightly acidic conditions [[protonation]] of the nitrogen occurs, changing the properties of histidine and the polypeptide as a whole. It is used by many proteins as a regulatory mechanism, changing the conformation and behavior of the polypeptide in acidic regions such as the late [[endosome]] or [[lysosome]], enforcing conformation change in enzymes. However only a few histidines are needed for this, so it is comparatively scarce. |
||
| − | |- |
||
| − | | I |
||
| − | | Ile |
||
| [[Isoleucine]] |
| [[Isoleucine]] |
||
| + | | Ile |
||
| − | | hydrophobic |
||
| − | | |
+ | | I |
| + | | nonpolar |
||
| − | | 6.05 |
||
| − | | |
+ | | neutral |
| − | | |
+ | | 4.5 |
| + | |- align="center" |
||
| − | | |
||
| + | | [[Leucine]] |
||
| − | | [[essential amino acid|Essential]] for humans. Isoleucine, leucine and valine have large aliphatic hydrophobic side chains. Their molecules are rigid, and their mutual hydrophobic interactions are important for the correct folding of proteins, as these chains tend to be located inside of the protein molecule. |
||
| + | | Leu |
||
| − | |- |
||
| − | | |
+ | | L |
| + | | nonpolar |
||
| − | | Lys |
||
| + | | neutral |
||
| + | | 3.8 |
||
| + | |- align="center" |
||
| [[Lysine]] |
| [[Lysine]] |
||
| + | | Lys |
||
| + | | K |
||
| + | | polar |
||
| basic |
| basic |
||
| − | | |
+ | | -3.9 |
| + | |- align="center" |
||
| − | | 9.60 |
||
| + | | [[Methionine]] |
||
| − | | 2.16 |
||
| − | | |
+ | | Met |
| − | | |
+ | | M |
| + | | nonpolar |
||
| − | | [[essential amino acid|Essential]] for humans. Behaves similarly to arginine. Contains a long flexible side-chain with a positively-charged end. The flexibility of the chain makes lysine and arginine suitable for binding to molecules with many negative charges on their surfaces. E.g., [[deoxyribonucleic acid|DNA]]-binding proteins have their active regions rich with arginine and lysine. The strong charge makes these two amino acids prone to be located on the outer hydrophilic surfaces of the proteins; when they are found inside, they are usually paired with a corresponding negatively-charged amino acid, e.g., aspartate or glutamate. |
||
| + | | neutral |
||
| + | | 1.9 |
||
| + | |- align="center" |
||
| + | | [[Phenylalanine]] |
||
| + | | Phe |
||
| + | | F |
||
| + | | nonpolar |
||
| + | | neutral |
||
| + | | 2.8 |
||
| + | |- align="center" |
||
| + | | [[Proline]] |
||
| + | | Pro |
||
| + | | P |
||
| + | | nonpolar |
||
| + | | neutral |
||
| + | | -1.6 |
||
| + | |- align="center" |
||
| + | | [[Serine]] |
||
| + | | Ser |
||
| + | | S |
||
| + | | polar |
||
| + | | neutral |
||
| + | | -0.8 |
||
| + | |- align="center" |
||
| + | | [[Threonine]] |
||
| + | | Thr |
||
| + | | T |
||
| + | | polar |
||
| + | | neutral |
||
| + | | -0.7 |
||
| + | |- align="center" |
||
| + | | [[Tryptophan]] |
||
| + | | Trp |
||
| + | | W |
||
| + | | nonpolar |
||
| + | | neutral |
||
| + | | -0.9 |
||
| + | |- align="center" |
||
| + | | [[Tyrosine]] |
||
| + | | Tyr |
||
| + | | Y |
||
| + | | nonpolar |
||
| + | | neutral |
||
| + | | -1.3 |
||
| + | |- align="center" |
||
| + | | [[Valine]] |
||
| + | | Val |
||
| + | | V |
||
| + | | nonpolar |
||
| + | | neutral |
||
| + | | 4.2 |
||
| + | |} |
||
| + | |||
| + | In addition to the normal amino acid codes, placeholders were used historically in cases where [[Protein sequencing|chemical]] or [[X-ray crystallography|crystallographic]] analysis of a peptide or protein could not completely establish the identity of a certain residue in a structure. The ones they could not resolve between are these pairs of amino-acids: |
||
| + | |||
| + | {| class="wikitable" |
||
| + | ! Ambiguous Amino Acids |
||
| + | ! 3-Letter |
||
| + | ! 1-Letter |
||
| + | |- align="center" |
||
| + | | Asparagine or aspartic acid |
||
| + | | Asx |
||
| + | | B |
||
| + | |- align="center" |
||
| + | | Glutamine or glutamic acid |
||
| + | | Glx |
||
| + | | Z |
||
| + | |- align="center" |
||
| + | | Leucine or Isoleucine |
||
| + | | Xle |
||
| + | | J |
||
| + | |- align="center" |
||
| + | | Unspecified or unknown amino acid |
||
| + | | Xaa |
||
| + | | X |
||
| + | |} |
||
| + | |||
| + | '''Unk''' is sometimes used instead of '''Xaa''', but is less standard. |
||
| + | |||
| + | == Nonstandard amino acids == |
||
| + | [[Image:L-selenocysteine-2D-skeletal.png|thumb|200px|right|The amino acid [[selenocysteine]].]] |
||
| + | Aside from the twenty standard amino acids, there is a vast number of "nonstandard amino acids". Two of these can be encoded in the genetic code, but are rather rare in proteins. [[Selenocysteine]] is incorporated into some proteins at a UGA [[codon]], which is normally a stop codon.<ref>{{cite journal | author = Driscoll D, Copeland P | title = Mechanism and regulation of selenoprotein synthesis. | journal = Annu Rev Nutr | volume = 23 | issue = | pages = 17-40 | year = | pmid = 12524431}}</ref> [[Pyrrolysine]] is used by some [[methanogen]]ic [[archaea]] in [[enzyme]]s that they use to produce [[methane]]. It is coded for with the codon UAG.<ref>{{cite journal | author = Krzycki J | title = The direct genetic encoding of pyrrolysine. | journal = Curr Opin Microbiol | volume = 8 | issue = 6 | pages = 706-12 | year = 2005 | pmid = 16256420}}</ref> |
||
| + | |||
| + | Examples of nonstandard amino acids that are not found in [[proteins]] include [[lanthionine]], [[2-aminoisobutyric acid]], [[dehydroalanine]] and the [[neurotransmitter]] [[gamma-aminobutyric acid]]. Nonstandard amino acids often occur as intermediates in the [[metabolic pathway]]s for standard amino acids - for example [[ornithine]] and [[citrulline]] occur in the [[urea cycle]], part of amino acid [[catabolism]].<ref>{{cite journal |author=Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L |title=Almost all about citrulline in mammals |journal=Amino Acids |volume=29 |issue=3 |pages=177-205 |year=2005 |pmid=16082501}}</ref> |
||
| + | |||
| + | Nonstandard amino acids are usually formed through modifications to standard amino acids. For example, [[homocysteine]] is formed through the [[transsulfuration pathway]] or by the demethylation of methionine via the intermediate metabolite [[S-adenosyl methionine]], <ref>{{cite journal |author=Brosnan J, Brosnan M |title=The sulfur-containing amino acids: an overview |journal=J Nutr |volume=136 |issue=6 Suppl |pages=1636S-1640S |year=2006 |pmid=16702333}}</ref> while dopamine is synthesized from l-DOPA, and [[hydroxyproline]] is made by a [[posttranslational modification]] of [[proline]].<ref>{{cite journal |author=Kivirikko K, Pihlajaniemi T |title=Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases |journal=Adv Enzymol Relat Areas Mol Biol |volume=72 |issue= |pages=325-98 |year= |pmid=9559057}}</ref> |
||
| + | |||
| + | == Uses in technology == |
||
| + | |||
| + | {| class="wikitable" style="margin-left: auto; margin-right: auto;" |
||
| + | !Amino acid derivative |
||
| + | !Use in industry |
||
|- |
|- |
||
| + | |align="center" |[[Aspartame]] (aspartyl-phenylalanine-1-methyl ester) |
||
| − | | L |
||
| + | |align="center" |Low-calorie [[artificial sweetener]] |
||
| − | | Leu |
||
| − | | [[Leucine]] |
||
| − | | hydrophobic |
||
| − | | 131.17 |
||
| − | | 6.01 |
||
| − | | 2.33 |
||
| − | | 9.74 |
||
| − | | |
||
| − | | [[essential amino acid|Essential]] for humans. Behaves similar to isoleucine and valine. See isoleucine. |
||
|- |
|- |
||
| + | |align="center" |[[5-HTP]] (5-hydroxytryptophan) |
||
| − | | M |
||
| + | |align="center" |Treatment for depression and the neurological problems of [[phenylketonuria]]. |
||
| − | | Met |
||
| − | | [[Methionine]] |
||
| − | | hydrophobic |
||
| − | | 149.21 |
||
| − | | 5.74 |
||
| − | | 2.13 |
||
| − | | 9.28 |
||
| − | | |
||
| − | | [[essential amino acid|Essential]] for humans. Always the first amino acid to be incorporated into a protein; sometimes removed after translation. Like cysteine, contains sulfur, but with a methyl group instead of hydrogen. This methyl group can be activated, and is used in many reactions where a new carbon atom is being added to another molecule. |
||
|- |
|- |
||
| + | |align="center" |[[L-DOPA]] (L-dihydroxyphenylalanine) |
||
| − | | N |
||
| + | |align="center" |Treatment for [[Parkinsonism]]. |
||
| − | | Asn |
||
| + | |- |
||
| + | |align="center" |[[Monosodium glutamate]] |
||
| + | |align="center" |[[Food additive]] that enhances flavor. Confers the taste [[umami]]. |
||
| + | |} |
||
| + | |||
| + | ==Nutritional importance== |
||
| + | {{further|[[Protein in nutrition]]}} |
||
| + | |||
| + | Of the 20 standard proteinogenic amino acids, 10 are called [[essential amino acid]]s because the [[human body]] cannot [[synthesize]] them from other [[chemical compound|compounds]] through [[chemical reaction]]s, and they therefore must be obtained from food. [[Cysteine]], [[tyrosine]], [[histidine]] and [[arginine]] are considered as semiessential amino acids in children, because the metabolic pathways that synthesize these amino acids are not fully developed.<ref>{{cite journal |author=Imura K, Okada A |title=Amino acid metabolism in pediatric patients |journal=Nutrition |volume=14 |issue=1 |pages=143-8 |year=1998 |pmid=9437700}}</ref> |
||
| + | |||
| + | {| class="wikitable" |
||
| + | ! Essential |
||
| + | ! Nonessential |
||
| + | |- |
||
| + | | [[Isoleucine]] |
||
| + | | [[Alanine]] |
||
| + | |- |
||
| + | | [[Leucine]] |
||
| [[Asparagine]] |
| [[Asparagine]] |
||
| − | | hydrophilic |
||
| − | | 132.12 |
||
| − | | 5.41 |
||
| − | | 2.14 |
||
| − | | 8.72 |
||
| − | | |
||
| − | | Neutralized version of aspartic acid. |
||
|- |
|- |
||
| + | | [[Lysine]] |
||
| − | | P |
||
| + | | [[Aspartate]] |
||
| − | | Pro |
||
| − | | [[Proline]] |
||
| − | | hydrophobic |
||
| − | | 115.13 |
||
| − | | 6.30 |
||
| − | | 1.95 |
||
| − | | 10.64 |
||
| − | | |
||
| − | | Contains an unusual ring to the N-end amine group, which forces the CO-NH amide sequence into a fixed conformation. Can disrupt protein folding structures like [[alpha helix|α helix]] or [[beta sheet|β sheet]], forcing the desired kink in the protein chain. Common in [[collagen]], where it undergoes a [[posttranslational modification]] to [[hydroxyproline]]. Uncommon elsewhere. |
||
|- |
|- |
||
| + | | [[Methionine]] |
||
| − | | Q |
||
| + | | [[Cysteine]] |
||
| − | | Gln |
||
| − | | [[Glutamine]] |
||
| − | | hydrophilic |
||
| − | | 146.15 |
||
| − | | 5.65 |
||
| − | | 2.17 |
||
| − | | 9.13 |
||
| − | | |
||
| − | | Neutralized version of glutamic acid. Used in proteins and as a storage for [[ammonia]]. |
||
|- |
|- |
||
| + | | [[Phenylalanine]] |
||
| − | | R |
||
| + | | [[Glutamate]] |
||
| − | | Arg |
||
| − | | [[Arginine]] |
||
| − | | basic |
||
| − | | 174.20 |
||
| − | | 10.76 |
||
| − | | 1.82 |
||
| − | | 8.99 |
||
| − | | 12.48 |
||
| − | | Functionally similar to lysine. |
||
|- |
|- |
||
| − | | S |
||
| − | | Ser |
||
| − | | [[Serine]] |
||
| − | | hydrophilic |
||
| − | | 105.09 |
||
| − | | 5.68 |
||
| − | | 2.19 |
||
| − | | 9.21 |
||
| − | | |
||
| − | | Serine and threonine have a short group ended with a [[hydroxyl]] group. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in enzymes. Both are very hydrophylic, therefore the outer regions of soluble proteins tend to be rich with them. |
||
| − | |- |
||
| − | | T |
||
| − | | Thr |
||
| [[Threonine]] |
| [[Threonine]] |
||
| + | | [[Glutamine]] |
||
| − | | hydrophilic |
||
| + | |- |
||
| − | | 119.12 |
||
| + | | [[Tryptophan]] |
||
| − | | 5.60 |
||
| + | | [[Glycine]] |
||
| − | | 2.09 |
||
| − | | 9.10 |
||
| − | | |
||
| − | | [[essential amino acid|Essential]] for humans. Behaves similarly to serine. |
||
|- |
|- |
||
| − | | V |
||
| − | | Val |
||
| [[Valine]] |
| [[Valine]] |
||
| + | | [[Proline]] |
||
| − | | hydrophobic |
||
| − | | 117.15 |
||
| − | | 6.00 |
||
| − | | 2.39 |
||
| − | | 9.74 |
||
| − | | |
||
| − | | [[essential amino acid|Essential]] for humans. Behaves similarly to isoleucine and leucine. See isoleucine. |
||
|- |
|- |
||
| + | | [[Arginine]]* |
||
| − | | W |
||
| + | | [[Serine]] |
||
| − | | Trp |
||
| − | | [[Tryptophan]] |
||
| − | | hydrophobic |
||
| − | | 204.23 |
||
| − | | 5.89 |
||
| − | | 2.46 |
||
| − | | 9.41 |
||
| − | | |
||
| − | | [[essential amino acid|Essential]] for humans. Behaves similarly to phenylalanine and tyrosine (see phenylalanine). Precursor of [[serotonin]]. |
||
|- |
|- |
||
| + | | [[Histidine]]* |
||
| − | | Y |
||
| − | | Tyr |
||
| [[Tyrosine]] |
| [[Tyrosine]] |
||
| − | | hydrophobic |
||
| − | | 181.19 |
||
| − | | 5.64 |
||
| − | | 2.20 |
||
| − | | 9.21 |
||
| − | | 10.46 |
||
| − | | Behaves similarly to phenylalanine and tryptophan (see phenylalanine). Precursor of [[melanin]], [[epinephrine]], and [[thyroid hormone]]s. |
||
|} |
|} |
||
| + | (*) Essential only in certain cases |
||
| + | Several common [[mnemonic]]s have evolved for remembering the essential amino acids. PVT TIM HALL ("[[Private (rank)|Private]] Tim Hall") uses the first letter of each essential amino acid, excluding arginine.<ref name="timhall">Distance Medical Biochemistry course from the University of New England. http://www.faculty.une.edu/com/courses/bionut/distbio/obj-512/Chap39-pvttimhall.htm Access date [[25 February]] [[2006]]</ref> Another mnemonic that frequently occurs in student practice materials is "'''T'''hese '''t'''en '''v'''aluable '''a'''mino acids '''h'''ave '''l'''ong '''p'''reserved '''l'''ife '''i'''n '''m'''an".<ref name="valuableaminoacids">Memory aids for medical biochemistry. http://mednote.co.kr/Yellownote/BIOCHMNEMON.htm Access date [[25 February]] [[2006]]</ref> |
||
| + | == See also == |
||
| − | {| border="1" bordercolor="black" cellspacing="0" cellpadding="2" |
||
| − | |- |
||
| − | ! Amino acid |
||
| − | ! Abbrev. |
||
| − | ! Side chain |
||
| − | ! Hydro- phobic |
||
| − | ! Polar |
||
| − | ! [[Electric charge|Charged]] |
||
| − | ! Small |
||
| − | ! Tiny |
||
| − | ! [[Aromaticity|Aromatic]] or [[Aliphatic]] |
||
| − | ! [[van der Waals]] volume |
||
| − | | align="center" | '''[[Genetic code|Codon]]''' |
||
| − | | align="center" | '''Occurrence in proteins (%)''' |
||
| − | |- align="center" |
||
| − | | align="left" | [[Alanine]] |
||
| − | | Ala, A |
||
| − | | -CH<sub>3</sub> |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | X |
||
| − | | X |
||
| − | | - |
||
| − | | align="center" | 67 |
||
| − | | GCU, GCC, GCA, GCG |
||
| − | | 7.8 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Cysteine]] |
||
| − | | Cys, C |
||
| − | | -CH<sub>2</sub>[[Sulfur|S]]H |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 86 |
||
| − | | UGU, UGC |
||
| − | | align="center" | 1.9 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Aspartate]] |
||
| − | | Asp, D |
||
| − | | -CH<sub>2</sub>COOH |
||
| − | | - |
||
| − | | X |
||
| − | | negative |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 91 |
||
| − | | GAU, GAC |
||
| − | | align="center" | 5.3 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Glutamate]] |
||
| − | | Glu, E |
||
| − | | -CH<sub>2</sub>CH<sub>2</sub>COOH |
||
| − | | - |
||
| − | | X |
||
| − | | negative |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 109 |
||
| − | | GAA, GAG |
||
| − | | align="center" | 6.3 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Phenylalanine]] |
||
| − | | Phe, F |
||
| − | | -CH<sub>2</sub>C<sub>6</sub>H<sub>5</sub> |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | [[Aromaticity|Aromatic]] |
||
| − | | align="center" | 135 |
||
| − | | UUU, UUC |
||
| − | | 3.9 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Glycine]] |
||
| − | | Gly, G |
||
| − | | -H |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | X |
||
| − | | X |
||
| − | | - |
||
| − | | align="center" | 48 |
||
| − | | GGU, GGC, GGA, GGG |
||
| − | | 7.2 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Histidine]] |
||
| − | | His, H |
||
| − | | -CH<sub>2</sub>-[[imidazole|C<sub>3</sub>H<sub>3</sub>N<sub>2</sub>]] |
||
| − | | - |
||
| − | | X |
||
| − | | positive |
||
| − | | - |
||
| − | | - |
||
| − | | [[Aromaticity|Aromatic]] |
||
| − | | align="center" | 118 |
||
| − | | CAU, CAC |
||
| − | | 2.3 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Isoleucine]] |
||
| − | | Ile, I |
||
| − | | -CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>3</sub> |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | [[Aliphatic]] |
||
| − | | align="center" | 124 |
||
| − | | AUU, AUC, AUA |
||
| − | | 5.3 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Lysine]] |
||
| − | | Lys, K |
||
| − | | -(CH<sub>2</sub>)<sub>4</sub>NH<sub>2</sub> |
||
| − | | - |
||
| − | | X |
||
| − | | positive |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 135 |
||
| − | | AAA, AAG |
||
| − | | 5.9 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Leucine]] |
||
| − | | Leu, L |
||
| − | | -CH<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub> |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | [[Aliphatic]] |
||
| − | | align="center" | 124 |
||
| − | | UUA, UUG, CUU, CUC, CUA, CUG |
||
| − | | 9.1 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Methionine]] |
||
| − | | Met, M |
||
| − | | -CH<sub>2</sub>CH<sub>2</sub>[[Sulfur|S]]CH<sub>3</sub> |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 124 |
||
| − | | AUG |
||
| − | | 2.3 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Asparagine]] |
||
| − | | Asn, N |
||
| − | | -CH<sub>2</sub>CONH<sub>2</sub> |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 96 |
||
| − | | AAU, AAC |
||
| − | | 4.3 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Proline]] |
||
| − | | Pro, P |
||
| − | | -CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>- |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 90 |
||
| − | | CCU, CCC, CCA, CCG |
||
| − | | 5.2 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Glutamine]] |
||
| − | | Gln, Q |
||
| − | | -CH<sub>2</sub>CH<sub>2</sub>CONH<sub>2</sub> |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 114 |
||
| − | | CAA, CAG |
||
| − | | 4.2 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Arginine]] |
||
| − | | Arg, R |
||
| − | | -(CH<sub>2</sub>)<sub>3</sub>NH-C(NH)NH<sub>2</sub> |
||
| − | | - |
||
| − | | X |
||
| − | | positive |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 148 |
||
| − | | CGU, CGC, CGA, CGG, AGA, AGG |
||
| − | | 5.1 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Serine]] |
||
| − | | Ser, S |
||
| − | | -CH<sub>2</sub>OH |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | X |
||
| − | | X |
||
| − | | - |
||
| − | | align="center" | 73 |
||
| − | | UCU, UCC, UCA, UCG, AGU,AGC |
||
| − | | 6.8 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Threonine]] |
||
| − | | Thr, T |
||
| − | | -CH(OH)CH<sub>3</sub> |
||
| − | | X |
||
| − | | X |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | align="center" | 93 |
||
| − | | ACU, ACC, ACA, ACG |
||
| − | | 5.9 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Valine]] |
||
| − | | Val, V |
||
| − | | -CH(CH<sub>3</sub>)<sub>2</sub> |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | X |
||
| − | | - |
||
| − | | [[Aliphatic]] |
||
| − | | align="center" | 105 |
||
| − | | GUU, GUC, GUA, GUG |
||
| − | | 6.6 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Tryptophan]] |
||
| − | | Trp, W |
||
| − | | -CH<sub>2</sub>[[indole|C<sub>8</sub>H<sub>6</sub>N]] |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | [[Aromaticity|Aromatic]] |
||
| − | | align="center" | 163 |
||
| − | | UGG |
||
| − | | 1.4 |
||
| − | |- align="center" |
||
| − | | align="left" | [[Tyrosine]] |
||
| − | | Tyr, Y |
||
| − | | -CH<sub>2</sub>-C<sub>6</sub>H<sub>4</sub>OH |
||
| − | | X |
||
| − | | X |
||
| − | | - |
||
| − | | - |
||
| − | | - |
||
| − | | [[Aromaticity|Aromatic]] |
||
| − | | align="center" | 141 |
||
| − | | UAU, UAC |
||
| − | | 3.2 |
||
| − | |} |
||
| + | * [[Biochemistry]] |
||
| − | == Hydrophilic and hydrophobic amino acids == |
||
| + | * [[Beta amino acid]] |
||
| − | Depending on how [[polar molecule|polar]] the side chain, aminoacids can be [[hydrophilic]] or [[hydrophobic]] to various degree. This influences their interaction with other structures, both within the protein itself and within other proteins. The distribution of hydrophilic and hydrophobic aminoacids determines the [[tertiary structure]] of the protein, and their physical location on the outside structure of the proteins influences their [[quaternary structure]]. For example, soluble proteins have surfaces rich with polar aminoacids like [[serine]] and [[threonine]], while [[integral membrane protein]]s tend to have outer ring of hydrophobic aminoacids that anchors them to the [[lipid bilayer]], and proteins anchored to the membrane have a hydrophobic end that locks into the membrane. Similarly, proteins that have to bind to positive-charged molecules have surfaces rich with negatively charged aminoacids like glutamate and aspartate, while proteins binding to negative-charged molecules have surfaces rich with positively charged chains like lysine and arginine. |
||
| + | * [[Dietary supplements]] |
||
| + | * [[DOPA]] |
||
| + | * [[Gamma aminobutyric acid]] |
||
| + | * [[Glutamic acid]] |
||
| + | * [[Glucogenic amino acid]] |
||
| + | * [[Genetic code#RNA codon table|Table of codons]], 3-nucleotide sequences that encode each amino acid |
||
| + | * [[List of standard amino acids]] |
||
| + | * [[Nerve growth factor]] |
||
| + | ==References and notes== |
||
| + | <div class="references-small" style="-moz-column-count:2; column-count:2;"> |
||
| + | <references/> |
||
| + | </div> |
||
| + | ==Further reading== |
||
| − | Hydrophilic and hydrophobic interactions of the proteins do not have to rely only on aminoacids themselves. By various [[posttranslational modification]]s other chains can be attached to the proteins, forming hydrophobic [[lipoprotein]]s or hydrophylic [[glycoprotein]]s. |
||
| + | * Doolittle, R.F. (1989) Redundancies in protein sequences. In ''Predictions of Protein Structure and the Principles of Protein Conformation'' (Fasman, G.D. ed) Plenum Press, New York, pp. 599-623 |
||
| − | == Nonstandard amino acids == |
||
| + | * David L. Nelson and Michael M. Cox, ''Lehninger Principles of Biochemistry'', 3rd edition, 2000, Worth Publishers, ISBN 1-57259-153-6 |
||
| − | Aside from the twenty standard amino acids and the two special amino acids, [[selenocysteine]] and [[pyrrolysine]], already mentioned above, there is a vast number of "nonstandard amino acids" which are not used in the body's regular manufacturing of proteins. Examples of nonstandard amino acids include the [[sulfur]]-containing [[taurine]] and the neurotransmitters [[GABA]] and [[dopamine]]. Other examples are [[lanthionine]], [[1-amino isobutyric acid]], [[dehydroalanine]], [[dehydro-amino-butyric acid]], |
||
| + | == External links == |
||
| − | Nonstandard amino acids are usually formed through modifications to standard amino acids. For example, taurine can be formed by the [[decarboxylation]] of cysteine, while dopamine is synthesized from tyrosine and [[hydroxyproline]] is made by a [[posttranslational modification]] from [[proline]]. |
||
| + | * [http://www.chem.qmul.ac.uk/iupac/AminoAcid/ Nomenclature and Symbolism for Amino Acids and Peptides] International Union of Pure and Applied Chemistry and The International Union of Biochemistry and Molecular Biology. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) |
||
| − | ==Uses of substances derived from amino acids== |
||
| + | * [http://micro.magnet.fsu.edu/aminoacids/index.html Molecular Expressions: The Amino Acid Collection] - Has detailed information and microscopy photographs of each amino acid. |
||
| − | * [[Aspartame]] (aspartyl-phenylalanine-1-methyl ester) is an artificial sweetener. |
||
| + | * [http://researchnews.osu.edu/archive/aminoacd.htm 22nd amino acid] - Press release from Ohio State claiming discovery of a 22nd amino acid. |
||
| − | * [[5-HTP]] (5-hydroxytryptophan) has been used to treat neurological problems associated with [[PKU]] (phenylketonuria), as well as depression (as an alternative to L-Tryptophan). |
||
| + | * [http://www.russell.embl.de/aas/ Amino acid properties] - Properties of the amino acids (a tool aimed mostly at molecular geneticists trying to understand the meaning of mutations) |
||
| − | * [[L-DOPA]] (L-dihydroxyphenylalanine) is a drug used to treat [[Parkinsonism]]. |
||
| + | * [http://www.organic-chemistry.org/synthesis/C1C/nitrogen/alpha-amino-acids2.shtm Synthesis of Amino Acids and Derivatives] |
||
| − | * [[Monosodium glutamate]] is a [[food additive]] to enhance flavor. |
||
| + | * [http://www.newscientist.com/article.ns?id=mg19025545.200&feedId=online-news_rss20 Right-handed amino acids were left behind] |
||
| + | * [http://www2.iq.usp.br/docente/gutz/Curtipot_.html Amino acid solutions pH, titration curves and distribution diagrams - freeware] |
||
| + | * [http://www.mathiasbader.de/studium/biology/index.php?lng=en Learn the 20 proteinogenic amino acids online] |
||
| + | {{AminoAcids}} |
||
| − | ==See also== |
||
| − | *[[Essential amino acids]] |
||
| − | *[[Strecker amino acid synthesis]] |
||
| − | ==References== |
||
| − | *Doolittle, R.F. (1989) Redundancies in protein sequences. In ''Predictions of Protein Structure and the Principles of Protein Conformation'' (Fasman, G.D. ed) Plenum Press, New York, pp. 599-623 |
||
| − | *David L. Nelson and Michael M. Cox, ''Lehninger Principles of Biochemistry'', 3rd edition, 2000, Worth Publishers, ISBN 1572591536 |
||
| − | * [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T36-3XB0N6H-H&_coverDate=09%2F10%2F1999&_alid=241945989&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=4938&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=3cb10a335716303532fc517906a12b3a On the hydrophobic nature of cysteine.] |
||
| + | [[Category:Amino acids| ]] |
||
| − | ==External links== |
||
| − | * [http://micro.magnet.fsu.edu/aminoacids/index.html Molecular Expressions: The Amino Acid Collection] - Has detailed information and microscopy photographs of each amino acid. |
||
| − | *[http://researchnews.osu.edu/archive/aminoacd.htm 22nd amino acid] - Press release from Ohio State claiming discovery of a 22nd amino acid. |
||
| − | |||
| − | [[Category:Amino acids]] |
||
[[Category:Nitrogen metabolism]] |
[[Category:Nitrogen metabolism]] |
||
| + | [[Category:Dietary supplements]] |
||
| − | |||
| + | [[Category:Neurotransmitters]] |
||
| − | [[th:กรดอะมิโน]] |
||
| + | [[Category:Proteins]] |
||
| + | <!-- |
||
| + | [[ar:حمض أميني]] |
||
| + | [[bn:অ্যামিনো অ্যাসিড]] |
||
| + | [[bs:Aminokiselina]] |
||
[[bg:Аминокиселина]] |
[[bg:Аминокиселина]] |
||
[[ca:Aminoàcid]] |
[[ca:Aminoàcid]] |
||
| − | [[cs: |
+ | [[cs:Aminokyseliny]] |
[[da:Aminosyre]] |
[[da:Aminosyre]] |
||
[[de:Aminosäuren]] |
[[de:Aminosäuren]] |
||
[[et:Aminohapped]] |
[[et:Aminohapped]] |
||
| + | [[el:Αμινοξύ]] |
||
[[es:Aminoácido]] |
[[es:Aminoácido]] |
||
[[eo:Aminoacido]] |
[[eo:Aminoacido]] |
||
| + | [[eu:Aminoazido]] |
||
[[fa:اسیدهای آمینه]] |
[[fa:اسیدهای آمینه]] |
||
| + | [[fo:Aminosýra]] |
||
[[fr:Acide aminé]] |
[[fr:Acide aminé]] |
||
[[gl:Aminoácido]] |
[[gl:Aminoácido]] |
||
[[ko:아미노산]] |
[[ko:아미노산]] |
||
| + | [[hr:Aminokiselina]] |
||
[[io:Amin-acido]] |
[[io:Amin-acido]] |
||
| + | [[id:Asam amino]] |
||
[[it:Amminoacidi]] |
[[it:Amminoacidi]] |
||
[[he:חומצת אמינו]] |
[[he:חומצת אמינו]] |
||
| + | [[ka:ამინომჟავა]] |
||
| − | [[lt:Aminorūgštis]] |
||
| + | [[ku:Tirşiyên emînî]] |
||
| + | [[la:Acidum aminicum]] |
||
[[lv:Aminoskābe]] |
[[lv:Aminoskābe]] |
||
[[lb:Aminosaier]] |
[[lb:Aminosaier]] |
||
| + | [[lt:Aminorūgštis]] |
||
[[hu:Aminosav]] |
[[hu:Aminosav]] |
||
[[mk:Амино киселина]] |
[[mk:Амино киселина]] |
||
| Line 650: | Line 404: | ||
[[no:Aminosyre]] |
[[no:Aminosyre]] |
||
[[nn:Aminosyre]] |
[[nn:Aminosyre]] |
||
| − | [[ |
+ | [[nov:Amino-aside]] |
| + | [[oc:Aminoacid]] |
||
| + | [[om:Amino Acid]] |
||
| + | [[pl:Aminokwasy]] |
||
[[pt:Aminoácido]] |
[[pt:Aminoácido]] |
||
| + | [[ro:Aminoacizi]] |
||
[[ru:Аминокислоты]] |
[[ru:Аминокислоты]] |
||
| + | [[simple:Amino acid]] |
||
| + | [[sk:Aminokyselina]] |
||
[[sl:Aminokislina]] |
[[sl:Aminokislina]] |
||
[[sr:Аминокиселина]] |
[[sr:Аминокиселина]] |
||
| + | [[sh:Aminokiselina]] |
||
[[su:Asam amino]] |
[[su:Asam amino]] |
||
[[fi:Aminohappo]] |
[[fi:Aminohappo]] |
||
[[sv:Aminosyra]] |
[[sv:Aminosyra]] |
||
[[th:กรดอะมิโน]] |
[[th:กรดอะมิโน]] |
||
| + | [[vi:Axít amin]] |
||
[[tr:Aminoasit]] |
[[tr:Aminoasit]] |
||
| − | [[uk: |
+ | [[uk:Амінокислоти]] |
[[zh:氨基酸]] |
[[zh:氨基酸]] |
||
| + | --> |
||
| − | |||
{{enWP| Amino_acids}} |
{{enWP| Amino_acids}} |
||
Revision as of 19:25, 29 August 2007
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
Phenylalanine is one of the standard amino acids.
In chemistry, an amino acid is a molecule that contains both amine and carboxyl functional groups. In biochemistry, this term refers to alpha-amino acids with the general formula H2NCHRCOOH, where R is an organic substituent.[1] In the alpha amino acids, the amino and carboxylate groups are attached to the same carbon, which is called the α–carbon. The various alpha amino acids differ in which side chain (R group) is attached to their alpha carbon. They can vary in size from just a hydrogen atom in glycine, through a methyl group in alanine, to a large heterocyclic group in tryptophan.
Beyond the amino acids that recur throughout biochemistry (see below), many non-natural amino acids are also important. The chelating agents EDTA and nitriloacetic acid are alpha amino acids that are industrially synthesized (sometimes from naturally occurring amino acids).
Overview
Alpha-amino acids are the building blocks of proteins. A protein forms via the condensation of amino acids to form a chain of amino acid "residues" linked by peptide bonds. Proteins are defined by their unique sequence of amino acid residues; this sequence is the primary structure of the protein. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins.
About twenty standard amino acids are used by cells in protein biosynthesis, and these are specified by the general genetic code. These twenty amino acids are biosynthesized from other molecules, but organisms differ in which ones they can synthesize and which ones must be provided in their diet. The ones that cannot be synthesized by an organism are called essential amino acids.
Functions in proteins
- See also: Primary structure and Posttranslational modification
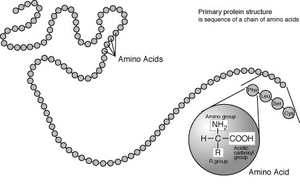
A polypeptide is a chain of amino acids.
Amino acids are the basic structural building units of proteins. They form short polymer chains called peptides or longer chains either called polypeptides or proteins. The process of such formation from an mRNA template is known as translation which is part of protein biosynthesis. Twenty amino acids are encoded by the standard genetic code and are called proteinogenic or standard amino acids. Other amino acids contained in proteins are usually formed by post-translational modification, which is modification after translation in protein synthesis. These modifications are often essential for the function or regulation of a protein; for example, the carboxylation of glutamate allows for better binding of calcium cations, and the hydroxylation of proline is critical for maintaining connective tissues and responding to oxygen starvation. Such modifications can also determine the localization of the protein, e.g., the addition of long hydrophobic groups can cause a protein to bind to a phospholipid membrane.
Non-protein functions
The twenty standard amino acids are either used to synthesize proteins and other biomolecules, or oxidized to urea and carbon dioxide as a source of energy.[2] The oxidation pathway starts with the removal of the amino group by a transaminase, the amino group is then fed into the urea cycle. The other product of transamidation is a keto acid that enters the citric acid cycle.[3] Glucogenic amino acids can also be converted into glucose, through gluconeogenesis.[4]
Hundreds of types of non-protein amino acids have been found in nature and they have multiple functions in living organisms. Microorganisms and plants can produce uncommon amino acids. In microbes, examples include 2-aminoisobutyric acid and lanthionine, which is a sulfide-bridged alanine dimer. Both these amino acids are both found in peptidic lantibiotics such as alamethicin.[5] While in plants, 1-Aminocyclopropane-1-carboxylic acid is a small disubstituted cyclic amino acid that is a key intermediate in the production of the plant hormone ethylene.[6]
In humans, non-protein amino acids also have biologically-important roles. Glycine, gamma-aminobutyric acid and glutamate are neurotransmitters and many amino acids are used to synthesize other molecules, for example:
- Tryptophan is a precursor of the neurotransmitter serotonin
- Glycine is a precursor of porphyrins such as heme
- Arginine is a precursor of nitric oxide
- Carnitine is used in lipid transport within a cell,
- Ornithine and S-adenosylmethionine are precursors of polyamines,
- Homocysteine is an intermediate in S-adenosylmethionine recycling
Also present are hydroxyproline, hydroxylysine, and sarcosine. The thyroid hormones are also alpha-amino acids.
Some amino acids have even been detected in meteorites, especially in a type known as carbonaceous chondrites.[7] This observation has prompted the suggestion that life may have arrived on earth from an extraterrestrial source.
General structure
- Further information: List of standard amino acids
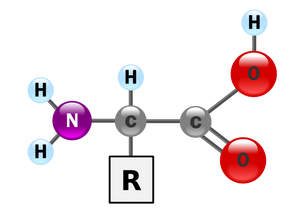
The general structure of an α-amino acid, with the amino group on the left and the carboxyl group on the right.
In the structure shown to the right, the R represents a side chain specific to each amino acid. The central carbon atom called Cα is a chiral central carbon atom (with the exception of glycine) to which the two termini and the R-group are attached. Amino acids are usually classified by the properties of the side chain into four groups. The side chain can make them behave like a weak acid, a weak base, a hydrophile if they are polar, and hydrophobe if they are nonpolar. The chemical structures of the 20 standard amino acids, along with their chemical properties, are cataloged in the list of standard amino acids.
The phrase "branched-chain amino acids" or BCAA is sometimes used to refer to the amino acids having aliphatic side-chains that are non-linear, these are leucine, isoleucine and valine. Proline is the only proteinogenic amino acid whose side group links to the α-amino group, and thus is also the only proteinogenic amino acid containing a secondary amine at this position. Proline has sometimes been termed an imino acid, but this is not correct in the current nomenclature.[8]
The two optical isomers of alanine.
Isomerism
Most amino acids can exist in either of two optical isomers, called D and L. The L-amino acids represent the vast majority of amino acids found in proteins. D-amino acids are found in some proteins produced by exotic sea-dwelling organisms, such as cone snails.[9] They are also abundant components of the peptidoglycan cell walls of bacteria.[10]
The L and D conventions for amino acid configuration do not refer to the optical activity, but rather to the optical activity of the isomer of glyceraldehyde having the same stereochemistry as the amino acid. S-Glyceraldehyde is levorotary, and R-glyceraldehyde is dexterorotary, and so S-amino acids are called L- even if they are not levorotary, and R-amino acids are likewise called D- even if they are not dexterorotary.
There are two exceptions to these general rules of amino acid isomerism. Firstly, glycine, where R = H, no isomerism is possible because the alpha-carbon bears two identical groups (hydrogen). Secondly, in cysteine, the L = S and D = R assignment is reversed to L = R and D = S. Cysteine is structured similarly (with respect to glyceraldehyde) to the other amino acids but the sulfur atom alters the interpretation of the Cahn-Ingold-Prelog priority rule.
Reactions
As amino acids have both a primary amine group and a primary carboxyl group, these chemicals can undergo most of the reactions associated with these functional groups. These include nucleophilic addition, amide bond formation and imine formation for the amine group and esterification, amide bond formation and decarboxylation for the carboxylic acid group. The multiple side chains of amino acids can also undergo chemical reactions. The types of these reactions are determined by the groups on these side chains and are discussed in the articles dealing with each specific type of amino acid.
Peptide bond formation

The condensation of two amino acids to form a peptide bond.
- For more details on this topic, see Peptide bond.
As both the amine and carboxylic acid groups of amino acids can react to form amide bonds, one amino acid molecule can react with another and become joined through an amide linkage. This polymerization of amino acids is what creates proteins. This condensation reaction yields the newly formed peptide bond and a molecule of water. In cells, this reaction does not occur directly, instead the amino acid is activated by attachment to a transfer RNA molecule through an ester bond. This aminoacyl-tRNA is produced in an ATP-dependent reaction carried out by an aminoacyl tRNA synthetase.[11] This aminoacyl-tRNA is then a substrate for the ribosome, which catalyzes the attack of the amino group of the elongating protein chain on the ester bond.[12] As a result of this mechanism, all proteins are synthesized starting at their N-terminus and moving towards their C-terminus.
However, not all peptide bonds are formed in this way. In a few cases peptides are synthesized by specific enzymes. For example, the tripeptide glutathione is an essential part of the defenses of cells against oxidative stress. This peptide is synthesized in two steps from free amino acids.[13] In the first step gamma-glutamylcysteine synthetase condenses cysteine and glutamic acid through a peptide bond formed between the side-chain carboxyl of the glutamate (the gamma carbon of this side chain) and the amino group of the cysteine. This dipeptide is then condensed with glycine by glutathione synthetase to form glutathione.[14]
In chemistry, peptides are synthesized by a variety of reactions. One of the most used in solid-phase peptide synthesis, which uses the aromatic oxime derivatives of amino acids as activated units. These are added in sequence onto the growing peptide chain, which is attached to a solid resin support.[15]
Zwitterions
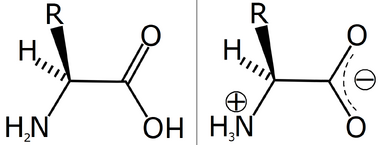
An amino acid, in its (1) normal (unionized) and (2) zwitterionic forms.
As amino acids have both the active groups of an amine and a carboxylic acid they can be considered both acid and base (though their natural pH is usually influenced by the R group). At a certain pH known as the isoelectric point, the amine group gains a positive charge (is protonated) and the acid group a negative charge (is deprotonated). The exact value is specific to each different amino acid. This ion is known as a zwitterion, which comes from the German word Zwitter meaning "hybrid". A zwitterion can be extracted from the solution as a white crystalline structure with a very high melting point, due to its dipolar nature. Near-neutral physiological pH allows most free amino acids to exist as zwitterions.
Hydrophilic and hydrophobic amino acids
Depending on the polarity of the side chain, amino acids vary in their hydrophilic or hydrophobic character. These properties are important in protein structure and protein-protein interactions. The importance of the physical properties of the side chains comes from the influence this has on the amino acid residues' interactions with other structures, both within a single protein and between proteins. The distribution of hydrophilic and hydrophobic amino acids determines the tertiary structure of the protein, and their physical location on the outside structure of the proteins influences their quaternary structure. For example, soluble proteins have surfaces rich with polar amino acids like serine and threonine, while integral membrane proteins tend to have outer ring of hydrophobic amino acids that anchors them into the lipid bilayer, and proteins anchored to the membrane have a hydrophobic end that locks into the membrane. Similarly, proteins that have to bind to positively-charged molecules have surfaces rich with negatively charged amino acids like glutamate and aspartate, while proteins binding to negatively-charged molecules have surfaces rich with positively charged chains like lysine and arginine. Recently a new scale of hydrophobicity based on the free energy of hydrophobic association has been proposed[16]
Hydrophilic and hydrophobic interactions of the proteins do not have to rely only on the sidechains of amino acids themselves. By various posttranslational modifications other chains can be attached to the proteins, forming hydrophobic lipoproteins or hydrophilic glycoproteins.
Table of standard amino acid abbreviations and side chain properties
- Main article: List of standard amino acids
| Amino Acid | 3-Letter | 1-Letter | Side chain polarity | Side chain acidity or basicity | Hydropathy index[17] |
|---|---|---|---|---|---|
| Alanine | Ala | A | nonpolar | neutral | 1.8 |
| Arginine | Arg | R | polar | basic (strongly) | -4.5 |
| Asparagine | Asn | N | polar | neutral | -3.5 |
| Aspartic acid | Asp | D | polar | acidic | -3.5 |
| Cysteine | Cys | C | polar | neutral | 2.5 |
| Glutamic acid | Glu | E | polar | acidic | -3.5 |
| Glutamine | Gln | Q | polar | neutral | -3.5 |
| Glycine | Gly | G | nonpolar | neutral | -0.4 |
| Histidine | His | H | polar | basic (weakly) | -3.2 |
| Isoleucine | Ile | I | nonpolar | neutral | 4.5 |
| Leucine | Leu | L | nonpolar | neutral | 3.8 |
| Lysine | Lys | K | polar | basic | -3.9 |
| Methionine | Met | M | nonpolar | neutral | 1.9 |
| Phenylalanine | Phe | F | nonpolar | neutral | 2.8 |
| Proline | Pro | P | nonpolar | neutral | -1.6 |
| Serine | Ser | S | polar | neutral | -0.8 |
| Threonine | Thr | T | polar | neutral | -0.7 |
| Tryptophan | Trp | W | nonpolar | neutral | -0.9 |
| Tyrosine | Tyr | Y | nonpolar | neutral | -1.3 |
| Valine | Val | V | nonpolar | neutral | 4.2 |
In addition to the normal amino acid codes, placeholders were used historically in cases where chemical or crystallographic analysis of a peptide or protein could not completely establish the identity of a certain residue in a structure. The ones they could not resolve between are these pairs of amino-acids:
| Ambiguous Amino Acids | 3-Letter | 1-Letter |
|---|---|---|
| Asparagine or aspartic acid | Asx | B |
| Glutamine or glutamic acid | Glx | Z |
| Leucine or Isoleucine | Xle | J |
| Unspecified or unknown amino acid | Xaa | X |
Unk is sometimes used instead of Xaa, but is less standard.
Nonstandard amino acids
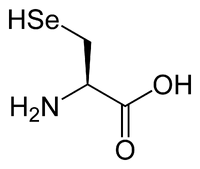
The amino acid selenocysteine.
Aside from the twenty standard amino acids, there is a vast number of "nonstandard amino acids". Two of these can be encoded in the genetic code, but are rather rare in proteins. Selenocysteine is incorporated into some proteins at a UGA codon, which is normally a stop codon.[18] Pyrrolysine is used by some methanogenic archaea in enzymes that they use to produce methane. It is coded for with the codon UAG.[19]
Examples of nonstandard amino acids that are not found in proteins include lanthionine, 2-aminoisobutyric acid, dehydroalanine and the neurotransmitter gamma-aminobutyric acid. Nonstandard amino acids often occur as intermediates in the metabolic pathways for standard amino acids - for example ornithine and citrulline occur in the urea cycle, part of amino acid catabolism.[20]
Nonstandard amino acids are usually formed through modifications to standard amino acids. For example, homocysteine is formed through the transsulfuration pathway or by the demethylation of methionine via the intermediate metabolite S-adenosyl methionine, [21] while dopamine is synthesized from l-DOPA, and hydroxyproline is made by a posttranslational modification of proline.[22]
Uses in technology
| Amino acid derivative | Use in industry |
|---|---|
| Aspartame (aspartyl-phenylalanine-1-methyl ester) | Low-calorie artificial sweetener |
| 5-HTP (5-hydroxytryptophan) | Treatment for depression and the neurological problems of phenylketonuria. |
| L-DOPA (L-dihydroxyphenylalanine) | Treatment for Parkinsonism. |
| Monosodium glutamate | Food additive that enhances flavor. Confers the taste umami. |
Nutritional importance
- Further information: Protein in nutrition
Of the 20 standard proteinogenic amino acids, 10 are called essential amino acids because the human body cannot synthesize them from other compounds through chemical reactions, and they therefore must be obtained from food. Cysteine, tyrosine, histidine and arginine are considered as semiessential amino acids in children, because the metabolic pathways that synthesize these amino acids are not fully developed.[23]
| Essential | Nonessential |
|---|---|
| Isoleucine | Alanine |
| Leucine | Asparagine |
| Lysine | Aspartate |
| Methionine | Cysteine |
| Phenylalanine | Glutamate |
| Threonine | Glutamine |
| Tryptophan | Glycine |
| Valine | Proline |
| Arginine* | Serine |
| Histidine* | Tyrosine |
(*) Essential only in certain cases
Several common mnemonics have evolved for remembering the essential amino acids. PVT TIM HALL ("Private Tim Hall") uses the first letter of each essential amino acid, excluding arginine.[24] Another mnemonic that frequently occurs in student practice materials is "These ten valuable amino acids have long preserved life in man".[25]
See also
- Biochemistry
- Beta amino acid
- Dietary supplements
- DOPA
- Gamma aminobutyric acid
- Glutamic acid
- Glucogenic amino acid
- Table of codons, 3-nucleotide sequences that encode each amino acid
- List of standard amino acids
- Nerve growth factor
References and notes
- ↑ Proline is an exception to this general formula. It lacks the NH2 group because of the cyclization of the side chain.
- ↑ Sakami W, Harrington H. Amino acid metabolism. Annu Rev Biochem 32: 355-98.
- ↑ Brosnan J (2000). Glutamate, at the interface between amino acid and carbohydrate metabolism. J Nutr 130 (4S Suppl): 988S-90S.
- ↑ Young V, Ajami A (2001). Glutamine: the emperor or his clothes?. J Nutr 131 (9 Suppl): 2449S-59S; discussion 2486S-7S.
- ↑ Whitmore L, Wallace B (2004). Analysis of peptaibol sequence composition: implications for in vivo synthesis and channel formation.. Eur Biophys J 33 (3): 233-7.
- ↑ Alexander L, Grierson D (2002). Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53 (377): 2039-55.
- ↑ Llorca J (2004). Organic matter in meteorites.. Int Microbiol 7 (4): 239-48.
- ↑ Claude Liebecq (Ed) Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 39-69 ISBN 978-1855780057
- ↑ Pisarewicz K, Mora D, Pflueger F, Fields G, Marí F (2005). Polypeptide chains containing D-gamma-hydroxyvaline.. J Am Chem Soc 127 (17): 6207-15.
- ↑ van Heijenoort J (2001). Formation of the glycan chains in the synthesis of bacterial peptidoglycan.. Glycobiology 11 (3): 25R-36R.
- ↑ Ibba M, Söll D (2001). The renaissance of aminoacyl-tRNA synthesis. EMBO Rep 2 (5): 382-7.
- ↑ Lengyel P, Söll D (1969). Mechanism of protein biosynthesis. Bacteriol Rev 33 (2): 264-301.
- ↑ Wu G, Fang Y, Yang S, Lupton J, Turner N (2004). Glutathione metabolism and its implications for health. J Nutr 134 (3): 489-92.
- ↑ Meister A (1988). Glutathione metabolism and its selective modification. J Biol Chem 263 (33): 17205–8.
- ↑ Carpino, L. A. (1992) 1-Hydroxy-7-azabenzotriazole. An efficient Peptide Coupling Additive. J. Am. Chem. Soc. 115, 4397-4398.
- ↑ Urry, D. W. (2004). The change in Gibbs free energy for hydrophobic association - Derivation and evaluation by means of inverse temperature transitions. Chemical Physics Letters 399 (1-3): 177-183.
- ↑ Kyte J & RF Doolittle (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. (157): 105-132. PMID 7108955.
- ↑ Driscoll D, Copeland P. Mechanism and regulation of selenoprotein synthesis.. Annu Rev Nutr 23: 17-40.
- ↑ Krzycki J (2005). The direct genetic encoding of pyrrolysine.. Curr Opin Microbiol 8 (6): 706-12.
- ↑ Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, Cynober L (2005). Almost all about citrulline in mammals. Amino Acids 29 (3): 177-205.
- ↑ Brosnan J, Brosnan M (2006). The sulfur-containing amino acids: an overview. J Nutr 136 (6 Suppl): 1636S-1640S.
- ↑ Kivirikko K, Pihlajaniemi T. Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv Enzymol Relat Areas Mol Biol 72: 325-98.
- ↑ Imura K, Okada A (1998). Amino acid metabolism in pediatric patients. Nutrition 14 (1): 143-8.
- ↑ Distance Medical Biochemistry course from the University of New England. http://www.faculty.une.edu/com/courses/bionut/distbio/obj-512/Chap39-pvttimhall.htm Access date 25 February 2006
- ↑ Memory aids for medical biochemistry. http://mednote.co.kr/Yellownote/BIOCHMNEMON.htm Access date 25 February 2006
Further reading
- Doolittle, R.F. (1989) Redundancies in protein sequences. In Predictions of Protein Structure and the Principles of Protein Conformation (Fasman, G.D. ed) Plenum Press, New York, pp. 599-623
- David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry, 3rd edition, 2000, Worth Publishers, ISBN 1-57259-153-6
External links
- Nomenclature and Symbolism for Amino Acids and Peptides International Union of Pure and Applied Chemistry and The International Union of Biochemistry and Molecular Biology. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)
- Molecular Expressions: The Amino Acid Collection - Has detailed information and microscopy photographs of each amino acid.
- 22nd amino acid - Press release from Ohio State claiming discovery of a 22nd amino acid.
- Amino acid properties - Properties of the amino acids (a tool aimed mostly at molecular geneticists trying to understand the meaning of mutations)
- Synthesis of Amino Acids and Derivatives
- Right-handed amino acids were left behind
- Amino acid solutions pH, titration curves and distribution diagrams - freeware
- Learn the 20 proteinogenic amino acids online
| Alanine | Arginine | Asparagine | Aspartic acid | Cysteine | Glutamic acid | Glutamine | Glycine | Histidine | Isoleucine | Leucine | Lysine | Methionine | Phenylalanine | Proline | Serine | Threonine | Tryptophan | Tyrosine | Valine |
| Essential amino acid | Protein | Peptide | Genetic code |
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |