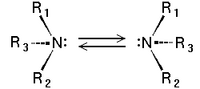No edit summary |
(→See also: spelling) |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
{{PsyPerspective}} |
{{PsyPerspective}} |
||
| + | [[Image:Amine-2D-general.png|thumb|150px|right|The general structure of an amine]] |
||
| − | [[image:ammonia.png|frame|Ammonia]] |
||
| − | '''Amines''' are organic compounds containing [[nitrogen]] as the key atom in the amine [[functional group]]. Amines have structures resembling [[ammonia]], where one or more [[hydrogen]] atoms are replaced by [[alkyl]] groups or other groups where the nitrogen is bonded to a carbon atom in the group (groups symbolized by '''R''' below). However, if any of the carbons bonded to the nitrogen that is part of a [[carbonyl group]], then the compound is considered an [[amide]] rather than an amine. |
||
| + | '''Amines''' are [[organic compound]]s and a type of [[functional group]] that contain [[nitrogen]] as the key atom. Structurally amines resemble [[ammonia]], wherein one or more [[hydrogen]] atoms are replaced by organic [[substituent]]s such as [[alkyl]] and [[aryl]] groups. An important exception to this rule is that compounds of the type RC(O)NR<sub>2</sub>, where the C(O) refers to a [[carbonyl group]], are called [[amide]]s rather than amines. Amides and amines have different structures and properties, so the distinction is [[chemical]]ly important. Somewhat confusing is the fact that amines in which an N-H group has been replaced by an N-M group (M = metal) are also called amides. Thus (CH<sub>3</sub>)<sub>2</sub>NLi is lithium dimethylamide. |
||
| ⚫ | |||
| + | Amines are central in organic chemistry, all life processes known, as the core part of [[amino acid]]s. |
||
| ⚫ | |||
| + | ==Introduction== |
||
| − | As shown in the following pictures, if only one of the hydrogens in ammonia is replaced by a carbon based group, then it is a '''primary amine'''. If two of the hydrogens are replaced by two carbon based groups, then it is a '''secondary amine'''. If all three hydrogens are replaced with three carbon based groups, then it is a '''tertiary amine'''. Note: the subscripts on the '''R''' groups are simply used to label these groups to differentiate them and show that they may be different (or they could be the same). However, the number subscripts on the H atoms show how many H atoms there are in that group. |
||
| + | ===[[Aliphatic]] Amines=== |
||
| − | {| border="1" |
||
| + | As displayed in the images below, '''primary amines''' arise when one of three hydrogen atoms in ammonia is replaced by an organic substituent. '''Secondary amines''' have two organic substituents bound to N together with one H. In '''tertiary amines''' all three hydrogen atoms are replaced by organic substituents. Note: the subscripts on the '''R''' groups are simply used to differentiate the organic substituents <!--superscripts are preferred-->. However, the number subscripts on the H atoms show how many H atoms there are in that group. It is also possible to have four alkyl substituents on the nitrogen. These compounds have a charged nitrogen center, and necessarily come with a negative counterion, so they are called quaternary [[ammonium]] salts. |
||
| − | |- valign="top" |
||
| + | {| class="wikitable" style="margin: 1em auto 1em auto" |
||
| − | | '''Primary Amine:'''<br />[[image:amina1.png]] |
||
| + | ! Primary amine || Secondary amine || Tertiary amine |
||
| − | | '''Secondary Amine:'''<br />[[image:amina2.png]] |
||
| ⚫ | |||
| − | | '''Tertiary Amine:'''<br />[[image:amina3.png]] |
||
| + | | <center>[[Image:Primary-amine-2D-general.png|100px|primary amine]]</center> || <center>[[Image:Secondary-amine-2D-general.png|100px|secondary amine]]</center> || <center>[[Image:Amine-2D-general.png|100px|tertiary amine]]</center> |
||
|} |
|} |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| + | {{main|Aromatic amines}} |
||
| ⚫ | |||
| + | |||
| − | {| border="1" |
||
| ⚫ | Aromatic amines have the nitrogen atom connected to an [[aromatic]] ring as in [[aniline]]s. The aromatic ring strongly decreases the [[base (chemistry)|basicity]] of the amine, depending on its substituents. Interestingly, the presence of an amine group strongly increases the reactivity of the aromatic ring, due to an electron-donating effect. One [[organic reaction]] involving aromatic amines is the [[Goldberg reaction]]. |
||
| + | |||
| ⚫ | |||
| + | * the prefix "N-" shows substitution on the nitrogen atom |
||
| + | * as prefix: "amino-" |
||
| + | * as suffix: "-amine" |
||
| + | * remember that chemical compounds are not proper nouns, so lower case is indicated throughout. |
||
| + | Systematic names for some common amines: |
||
| + | {| border="0" align="center" spacing="5" |
||
|- valign="top" align="center" |
|- valign="top" align="center" |
||
| Lower amines are named with the suffix ''-amine''.<br /> |
| Lower amines are named with the suffix ''-amine''.<br /> |
||
| − | [[ |
+ | [[Image:methylamine.png|100px]]<br /> |
| − | ''' |
+ | '''methylamine''' |
| Higher amines have the prefix ''amino'' as a functional group.<br /> |
| Higher amines have the prefix ''amino'' as a functional group.<br /> |
||
| − | [[ |
+ | [[Image:2-amino-pentane.png|150px]]<br /> |
| − | '''2- |
+ | '''2-aminopentane'''<br/>(or sometimes: ''pent-2-yl-amine'' or ''pentane-2-amine'') |
|} |
|} |
||
| + | *'''Primary amines''': |
||
| + | ** [[methylamine]] |
||
| + | ** [[ethanolamine]] or 2-aminoethanol |
||
| + | ** [[trisamine]] (or more commonly [[tris]]) (Its [[HCl]] salt is used as a [[pH]] [[buffering agent]] in [[biochemistry]]) |
||
| + | *'''Secondary amines''': |
||
| + | ** [[dimethylamine]] |
||
| + | ** [[methylethanolamine]] or 2-(methylamino)ethanol |
||
| + | ** [[Cyclic compound|Cyclic]] amines: |
||
| + | *** [[aziridine]] (3-member ring), |
||
| + | *** [[azetidine]] (4-member ring), |
||
| + | *** [[pyrrolidine]] (5-member ring) and |
||
| + | *** [[piperidine]] (6-member ring) |
||
| + | *'''Tertiary amines''': |
||
| + | ** [[trimethylamine]] |
||
| + | ** [[MDEA|methyldiethanolamine]] (MDEA) |
||
| + | ** [[dimethylethanolamine]] (DMEA) or 2-(dimethylamino)ethanol |
||
| + | ** [[bis-tris]] (It is used as a pH buffering agent in biochemistry) |
||
| + | ==Physical properties== |
||
| − | ==Properties== |
||
| + | ===General properties=== |
||
| + | # [[Hydrogen bonding]] significantly influences the properties of primary and secondary amines as well as the protonated derivatives of all amines. Thus the [[boiling point]] of amines is higher than those for the corresponding [[phosphine]]s, but generally lower than the corresponding [[alcohol]]s. Alcohols, or alkanols, resemble amines but feature an -OH group in place of NR<sub>2</sub>. Since oxygen is more [[electronegative]] than nitrogen, RO-''H'' is typically more acidic than the related R<sub>2</sub>N-''H'' compound. |
||
| + | # Methyl-, dimethyl-, trimethyl-, and [[ethylamine]] are gases under standard conditions, while [[diethylamine]] and [[triethylamine]] are liquids. Most other common alkyl amines are liquids; high [[molecular weight]] amines are, of course, solids. |
||
| + | # Gaseous amines possess a characteristic ammonia smell, liquid amines have a distinctive "fishy" smell. |
||
| + | # Most aliphatic amines display some solubility in water, reflecting their ability to form hydrogen bonds. Solubility decreases with the increase in the number of carbon atoms, especially when the carbon atom number is greater than 6. |
||
| + | # Aliphatic amines display significant solubility in organic [[solvent]]s, especially polar organic solvents. Primary amines react with [[ketone]]s such as [[acetone]], and most amines are incompatible with [[chloroform]] and [[carbon tetrachloride]]. |
||
| + | # The aromatic amines, such as [[aniline]], have their lone pair electrons [[conjugated system|conjugated]] into the benzene ring, thus their tendency to engage in hydrogen bonding is diminished. Otherwise they display the following properties: |
||
| + | #* Their boiling points are usually still high <!--"higher": than what?-->due to their larger size. |
||
| + | #* Diminished solubility in water, although they retain their solubility in suitable organic solvents only. |
||
| + | #* They are toxic <!-- which one has: b.p.184 C?--> and are easily absorbed through the skin: thus hazardous. |
||
| ⚫ | |||
| + | === Chirality=== |
||
| − | {| class="toccolours" style="float: right; border-collapse: collapse; margin: 0em 1em;" border="1" cellpadding="2" cellspacing="0" |
||
| + | Tertiary amines of the type NHRR' and NRR'R" are [[chirality (chemistry)|chiral]]: the nitrogen atom bears four distinct substituents counting the lone pair. The energy barrier for the [[nitrogen inversion|inversion]] of the stereocenter is relatively low, e.g. ~7 kcal/mol for a trialkylamine. The interconversion of the stereoisomers has been compared to the inversion of an open umbrella in to a strong wind. Because of this low barrier, amines such as NHRR' cannot be resolved optically and NRR'R" can only be resolved when the R, R', and R" groups are constrained in cyclic structures. |
||
| − | ! Ions of compound |
||
| − | ! K<sub>a</sub> (conjugate acid) |
||
| ⚫ | |||
| − | | [[Aniline]] C<sub>6</sub>H<sub>5</sub>-NH<sub>2</sub> |
||
| − | | 2.0·10<sup>-5</sup> M |
||
| − | |- |
||
| − | | [[Ammonia]] NH<sub>3</sub> |
||
| − | | 5.6·10<sup>-10</sup> M |
||
| − | |- |
||
| − | | [[Ethylene diamine]] NH<sub>2</sub>-CH<sub>2</sub>-CH<sub>2</sub>-NH<sub>2</sub> |
||
| − | | 1.3·10<sup>-10</sup> M |
||
| − | |- |
||
| − | | [[Butylamine]] CH<sub>3</sub>-CH<sub>2</sub>-CH<sub>2</sub>-CH<sub>2</sub>-NH<sub>2</sub> |
||
| − | | 0.15·10<sup>-10</sup> M |
||
| − | |} |
||
| + | == Biological activity == |
||
| − | Like ammonia, amines act as bases and are reasonably strong (see table for examples of conjugate acid K<sub>a</sub> values). The nitrogen atom has a [[lone electron pair]] available which can accept a H<sup>+</sup> ion to bond to the nitrogen forming a positive substituted ammonium ion. The pairs of dots on the N atoms in the chemical reactions shown in this article represent the lone electron pairs on the nitrogens in the amines. These lone pairs also contribute to the [[solubility]] of simple amines due to [[hydrogen bonding]] between [[water]] molecules and the lone electron pairs. |
||
| + | Amines have strong, characteristic, disagreeable odors, and are toxic. The smells of ammonia, fish, urine, rotting flesh and semen are all mainly composed of amines. Many kinds of biological activity produce amines by breakdown of [[amino acid]]s. |
||
| + | ===Drugs=== |
||
| − | <center>[[Image:Amine_to_Ammonium.PNG]]</center> |
||
| + | * [[Chlorpheniramine]] is an antihistamine that helps to relieve allergic disorders due to cold, hay fever, itchy skin, insect bites and stings. |
||
| − | |||
| + | * [[Chlorpromazine]] is a tranquillizer that sedates without inducing sleep. It is used to relieve anxiety, excitement, restlessness or even mental disorder. |
||
| − | Also, a [[halogenoalkane]] can react with an amine to form a corresponding alkyl-substituted amine, with the release of a halogen acid. |
||
| + | * [[Ephedrine]] and [[Phenylephrine]], as amine hydrochlorides, are used as decongestants. |
||
| − | |||
| + | * [[Amphetamine]], [[Methamphetamine]], and [[Methcathinone]] are amines that are listed as controlled substances by the [[Drug Enforcement Agency|DEA]]. |
||
| − | <center>[[Image:Alkylation_of_Amine.PNG]]</center> |
||
| + | * [[Amitriptyline]], [[Imipramine]], [[Lofepramine]] and [[Clomipramine]] are [[tricylic antidepressants]] and tertiary amines |
||
| − | |||
| + | * [[Nortriptyline]], [[Desipramine]], and [[Amoxapine]] are tricyclic antidepressants and secondary amines |
||
| − | If the reacting amine is a tertiary amine in such a reaction, then a positive [[quaternary ammonium cation]] will be formed along with a negative halide ion. |
||
| + | * (The tricylics are grouped by the nature of the final amine group on the side chain.) |
||
| − | |||
| − | <center>[[Image:Formation_of_Quat.PNG]]</center> |
||
| − | |||
| − | These sort of paired ion compounds are called '''[[quaternary ammonium salt]]s'''. The X shown in the above reactions can also be some other leaving group forming a corresponding acid or anion. |
||
| − | |||
| − | A tertiary amine molecule whose substituents are distinct is a chiral object: the nitrogen has four distinct substituents (counting the lone pair) with no plane of symmetry. However, the energetic barrier for the inversion of the stereocenter is relatively low (e.g. ~7 kcal/mol for a trialkylamine). As a result, interconversion of the stereoisomers is rapid and isolating stereospecific amines is very difficult. The effect has been compared to the inversion of an open umbrella due to a strong wind. |
||
| − | |||
| ⚫ | |||
| − | |||
| − | The [[volatile]] amines often have rotten fish smells. The resemblance is directly related; the bacterial decay of [[amino acid]]s in [[protein]] results in the production of amines. |
||
| − | |||
| − | == Synthesis == |
||
| − | Primary amines can be synthesized from ammonia and [[alkyl halide]]s by the [[Gabriel Synthesis]] or from [[azide]]s by the [[Staudinger reduction]]. [[Allyl]]ic amines can be prepared from [[imine]]s in the [[Aza-Baylis-Hillman reaction]] |
||
| − | |||
| − | == Reactions == |
||
| − | |||
| − | * Dissolving secondary and tertiary amines using strong acids like HI, HBr, or HCl does not lead to a lower grade amine and an alkylhalide, hence the nitrogen-group is not a preferred leaving group. |
||
| − | |||
| − | * The only kind of successful elimination is the elimination of an alkyl-group from [[quaternary ammonium salt]]s, the so called [[Hofmann Elimination]] |
||
| − | |||
| − | * Because they are basic, amines can neutralize [[carboxylic acid]]s to form the corresponding substituted ammonium carboxylate salts. Upon heating to 200<sup>o</sup> C, these salts will dehydrate to form [[amide]]s, if the initial amine was primary or secondary. |
||
| − | |||
| − | <center>[[Image:Amine_plus_Carboxylic_Acid.PNG]]</center> |
||
| − | |||
| − | * Derivatives of carboxylic acids, such as [[acyl chloride]]s, can react with primary or secondary amines to form [[amide]]s. |
||
| − | |||
| ⚫ | |||
| − | |||
| ⚫ | Aromatic amines |
||
== See also == |
== See also == |
||
| + | *[[Amitriptyline]] |
||
| − | |||
| + | *[[Atropine]] |
||
| ⚫ | |||
| + | *[[Bufotenine]] |
||
| + | *[[Chlordiazepoxide]] |
||
| + | *[[Chlorpromazine]] |
||
| + | *[[Chlorprothixene]] |
||
| + | *[[Cocaine]] |
||
| + | *[[Diphenhydramine]] |
||
| + | *[[Galanthamine]] |
||
| + | *[[Histamine]] |
||
| + | *[[Hydroxylamine]] |
||
| + | *[[Imipramine]] |
||
| + | *[[Mecamylamine]] |
||
| + | *[[Meperidine]] |
||
| + | *[[Methylphenidate]] |
||
| + | *[[Orphenadrine]] |
||
| + | *[[Phenethylamines]] |
||
| + | *[[Phenoxybenamine]] |
||
| + | *[[Physostigamine]] |
||
| + | *[[Puromycin]] |
||
| + | *[[Scopolamine]] |
||
| + | *[[Serotonin]] |
||
| + | *[[Thalidomide]] |
||
| + | *[[Trihexyphenyidyl]] |
||
| + | *[[Tryptamine]] |
||
| + | *[[Sympathomimetic amines]] |
||
| + | *[[Amino acids]] |
||
| ⚫ | |||
[[Category:Amines]] |
[[Category:Amines]] |
||
| + | [[Category:Sympathomimetic amines]] |
||
| − | |||
| + | [[Category:Amino acids]] |
||
<!-- |
<!-- |
||
Latest revision as of 08:20, 30 December 2008
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
The general structure of an amine
Amines are organic compounds and a type of functional group that contain nitrogen as the key atom. Structurally amines resemble ammonia, wherein one or more hydrogen atoms are replaced by organic substituents such as alkyl and aryl groups. An important exception to this rule is that compounds of the type RC(O)NR2, where the C(O) refers to a carbonyl group, are called amides rather than amines. Amides and amines have different structures and properties, so the distinction is chemically important. Somewhat confusing is the fact that amines in which an N-H group has been replaced by an N-M group (M = metal) are also called amides. Thus (CH3)2NLi is lithium dimethylamide.
Amines are central in organic chemistry, all life processes known, as the core part of amino acids.
See the Category:Amines for a list of types of amine and some real examples of this class of chemical.
Introduction
Aliphatic Amines
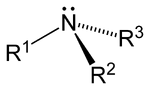
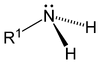
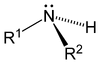
As displayed in the images below, primary amines arise when one of three hydrogen atoms in ammonia is replaced by an organic substituent. Secondary amines have two organic substituents bound to N together with one H. In tertiary amines all three hydrogen atoms are replaced by organic substituents. Note: the subscripts on the R groups are simply used to differentiate the organic substituents . However, the number subscripts on the H atoms show how many H atoms there are in that group. It is also possible to have four alkyl substituents on the nitrogen. These compounds have a charged nitrogen center, and necessarily come with a negative counterion, so they are called quaternary ammonium salts.
| Primary amine | Secondary amine | Tertiary amine |
|---|---|---|
 |
 |
 |
Similarly, an organic compound with multiple amino groups is called a diamine, triamine, tetraamine and so forth.
Aromatic amines
- Main article: Aromatic amines
Aromatic amines have the nitrogen atom connected to an aromatic ring as in anilines. The aromatic ring strongly decreases the basicity of the amine, depending on its substituents. Interestingly, the presence of an amine group strongly increases the reactivity of the aromatic ring, due to an electron-donating effect. One organic reaction involving aromatic amines is the Goldberg reaction.
Naming conventions
- the prefix "N-" shows substitution on the nitrogen atom
- as prefix: "amino-"
- as suffix: "-amine"
- remember that chemical compounds are not proper nouns, so lower case is indicated throughout.
Systematic names for some common amines:
| Lower amines are named with the suffix -amine. |
Higher amines have the prefix amino as a functional group.
|
- Primary amines:
- methylamine
- ethanolamine or 2-aminoethanol
- trisamine (or more commonly tris) (Its HCl salt is used as a pH buffering agent in biochemistry)
- Secondary amines:
- dimethylamine
- methylethanolamine or 2-(methylamino)ethanol
- Cyclic amines:
- aziridine (3-member ring),
- azetidine (4-member ring),
- pyrrolidine (5-member ring) and
- piperidine (6-member ring)
- Tertiary amines:
- trimethylamine
- methyldiethanolamine (MDEA)
- dimethylethanolamine (DMEA) or 2-(dimethylamino)ethanol
- bis-tris (It is used as a pH buffering agent in biochemistry)
Physical properties
General properties
- Hydrogen bonding significantly influences the properties of primary and secondary amines as well as the protonated derivatives of all amines. Thus the boiling point of amines is higher than those for the corresponding phosphines, but generally lower than the corresponding alcohols. Alcohols, or alkanols, resemble amines but feature an -OH group in place of NR2. Since oxygen is more electronegative than nitrogen, RO-H is typically more acidic than the related R2N-H compound.
- Methyl-, dimethyl-, trimethyl-, and ethylamine are gases under standard conditions, while diethylamine and triethylamine are liquids. Most other common alkyl amines are liquids; high molecular weight amines are, of course, solids.
- Gaseous amines possess a characteristic ammonia smell, liquid amines have a distinctive "fishy" smell.
- Most aliphatic amines display some solubility in water, reflecting their ability to form hydrogen bonds. Solubility decreases with the increase in the number of carbon atoms, especially when the carbon atom number is greater than 6.
- Aliphatic amines display significant solubility in organic solvents, especially polar organic solvents. Primary amines react with ketones such as acetone, and most amines are incompatible with chloroform and carbon tetrachloride.
- The aromatic amines, such as aniline, have their lone pair electrons conjugated into the benzene ring, thus their tendency to engage in hydrogen bonding is diminished. Otherwise they display the following properties:
- Their boiling points are usually still high due to their larger size.
- Diminished solubility in water, although they retain their solubility in suitable organic solvents only.
- They are toxic and are easily absorbed through the skin: thus hazardous.
Chirality
Tertiary amines of the type NHRR' and NRR'R" are chiral: the nitrogen atom bears four distinct substituents counting the lone pair. The energy barrier for the inversion of the stereocenter is relatively low, e.g. ~7 kcal/mol for a trialkylamine. The interconversion of the stereoisomers has been compared to the inversion of an open umbrella in to a strong wind. Because of this low barrier, amines such as NHRR' cannot be resolved optically and NRR'R" can only be resolved when the R, R', and R" groups are constrained in cyclic structures.
Biological activity
Amines have strong, characteristic, disagreeable odors, and are toxic. The smells of ammonia, fish, urine, rotting flesh and semen are all mainly composed of amines. Many kinds of biological activity produce amines by breakdown of amino acids.
Drugs
- Chlorpheniramine is an antihistamine that helps to relieve allergic disorders due to cold, hay fever, itchy skin, insect bites and stings.
- Chlorpromazine is a tranquillizer that sedates without inducing sleep. It is used to relieve anxiety, excitement, restlessness or even mental disorder.
- Ephedrine and Phenylephrine, as amine hydrochlorides, are used as decongestants.
- Amphetamine, Methamphetamine, and Methcathinone are amines that are listed as controlled substances by the DEA.
- Amitriptyline, Imipramine, Lofepramine and Clomipramine are tricylic antidepressants and tertiary amines
- Nortriptyline, Desipramine, and Amoxapine are tricyclic antidepressants and secondary amines
- (The tricylics are grouped by the nature of the final amine group on the side chain.)
See also
- Amitriptyline
- Atropine
- Bufotenine
- Chlordiazepoxide
- Chlorpromazine
- Chlorprothixene
- Cocaine
- Diphenhydramine
- Galanthamine
- Histamine
- Hydroxylamine
- Imipramine
- Mecamylamine
- Meperidine
- Methylphenidate
- Orphenadrine
- Phenethylamines
- Phenoxybenamine
- Physostigamine
- Puromycin
- Scopolamine
- Serotonin
- Thalidomide
- Trihexyphenyidyl
- Tryptamine
- Sympathomimetic amines
- Amino acids
- IUPAC nomenclature for the official naming rules for amines.
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |