(New page: {{BioPsy}} <!-- Here is a table of data; skip past it to edit the text. --> {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collap...) |
(-L tidy) |
||
| Line 104: | Line 104: | ||
|- |
|- |
||
|} |
|} |
||
| − | '''Acetaldehyde''', sometimes known as '''ethanal''', is an [[organic compound|organic chemical compound]] with the [[chemical formula|formula]] [[Carbon|C]][[Hydrogen|H]]<sub>3</sub>CH[[Oxygen|O]] or MeCHO. It is a flammable liquid with a fruity smell. Acetaldehyde occurs naturally in ripe [[fruit]], [[coffee]], and fresh |
+ | '''Acetaldehyde''', sometimes known as '''ethanal''', is an [[organic compound|organic chemical compound]] with the [[chemical formula|formula]] [[Carbon|C]][[Hydrogen|H]]<sub>3</sub>CH[[Oxygen|O]] or MeCHO. It is a flammable liquid with a fruity smell. Acetaldehyde occurs naturally in ripe [[fruit]], [[coffee]], and fresh bread and is produced by plants as part of their normal [[metabolism]]. It is probably best known as the chemical that causes "[[hangovers]]". |
In the chemical industry, acetaldehyde is used as an intermediate in the production of [[acetic acid]], certain [[ester]]s, and a number of other chemicals. In 1989, US production stood at 740 million pounds (336,000 t).{{Fact|date=February 2007}} |
In the chemical industry, acetaldehyde is used as an intermediate in the production of [[acetic acid]], certain [[ester]]s, and a number of other chemicals. In 1989, US production stood at 740 million pounds (336,000 t).{{Fact|date=February 2007}} |
||
==Ethenol== |
==Ethenol== |
||
| − | Only a trace of acetaldehyde exists as the |
+ | Only a trace of acetaldehyde exists as the enol form, [[ethenol]], with K<sub>eq</sub> = 6 x 10<sup>-5</sup>.<ref>March, J. “Organic Chemistry: Reactions, Mechanisms, and Structures” J. Wiley, New York: 1992. ISBN 0-471-58148-8.</ref> Ethenol has been detected in the interstellar medium. {{Fact|date=May 2007}} |
==Applications in organic synthesis== |
==Applications in organic synthesis== |
||
| − | Acetaldehyde is a common 2-carbon building block in |
+ | Acetaldehyde is a common 2-carbon building block in organic synthesis.<ref>Sowin, T. J.; Melcher, L. M. ”Acetaldehyde” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. {{DOI|10.1002/047084289}}</ref> Because of its small size and its availability as the anhydrous monomer (unlike formaldehyde), it is a common electrophile. With respect to its condensation reactions, acetaldehyde is [[prochiral]]. It is mainly used as a source of the CH<sub>3</sub>C<sup>+</sup>H(OH) [[synthon]] in [[aldol condensation|aldol]] and related condensation reactions.<ref>Behrens, C.; Paquette, L. A. “N-Benzyl-2,3-Azetidinedione” Organic Syntheses, Collected Volume 10, p.41 (2004)</ref> Grignard reagents and organolithium compounds react with MeCHO to give hydroxyethyl derivatives.<ref>Walter, L. A. “1-(α-Pyridyl)-2-Propanol” Organic Syntheses, Collected Volume 3, p.757 (1955).</ref> In one of the more spectacular condensation reactions, three equivalents of [[formaldehyde]] add to MeCHO to give [[pentaerythritol]], C(CH<sub>2</sub>OH)<sub>4</sub>.<ref>Schurink, H. B. J. “Pentaerythritol” Organic Syntheses, Collected Volume 1, p.425 (1941). http://www.orgsyn.org/orgsyn/pdfs/CV1P0425.pdf</ref> |
| − | In a [[Strecker amino acid synthesis|Strecker reaction]], acetaldehyde condenses with |
+ | In a [[Strecker amino acid synthesis|Strecker reaction]], acetaldehyde condenses with cyanide and ammonia to give, after [[hydrolysis]], the [[amino acid]] alanine.<ref>Kendall, E. C. McKenzie, B. F. “''dl''-Alanine” Organic Syntheses, Collected Volume 1, p.21 (1941). http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf</ref> Acetaldehyde can condense with amines to yield imines, such as the condensation with cyclohexylamine to give N-ethylidenecyclohexylamine. These imines can be used to direct subsequent reactions like an aldol condensation.<ref>[[Georg Wittig|Wittig, G.]]; Hesse, A. “Directed Aldol Condensations: β-Phenylcinnamaldehyde” Organic Syntheses, Collected Volume 6, p.901 (1988).</ref> |
| − | It is also an important building block for the synthesis of |
+ | It is also an important building block for the synthesis of heterocyclic compounds. A remarkable example is its conversion upon treatment with ammonia to 5-ethyl-2-methylpyridine ("aldehyde-collidine”).<ref>Frank, R. L.; Pilgrim, F. J.; Riener, E. F. “5-Ethyl-2-Methylpyridine” Organic Syntheses, Collected Volume 4, p. 451 (1963). http://www.orgsyn.org/orgsyn/pdfs/CV4P0451.pdf</ref> |
===Acetal derivatives=== |
===Acetal derivatives=== |
||
| Line 124: | Line 124: | ||
==Biological aspects== |
==Biological aspects== |
||
| − | In the [[liver]], the [[enzyme]] [[alcohol dehydrogenase]] converts ethanol into acetaldehyde, which is then further converted into harmless [[acetic acid]] by [[acetaldehyde dehydrogenase]]. The last steps of alcoholic |
+ | In the [[liver]], the [[enzyme]] [[alcohol dehydrogenase]] converts ethanol into acetaldehyde, which is then further converted into harmless [[acetic acid]] by [[acetaldehyde dehydrogenase]]. The last steps of alcoholic fermentation in bacteria, plants and yeast involve the conversion of [[pyruvate]] into acetaldehyde by the enzyme [[pyruvate decarboxylase]], followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehydrogenase, now operating in the opposite direction. |
===Acetaldehyde and hangovers=== |
===Acetaldehyde and hangovers=== |
||
| Line 132: | Line 132: | ||
==Other occurrences== |
==Other occurrences== |
||
| − | Acetaldehyde is an |
+ | Acetaldehyde is an air pollutant resulting from combustion, such as automotive exhaust and [[tobacco smoking|tobacco smoke]], contributing to the addictive properties of tobacco. <ref>''[http://www.britannica.com/eb/article-242792 Smoking]''. (2006). [[Encyclopædia Britannica]]. Accessed 27 Oct 2006.</ref> |
==Safety== |
==Safety== |
||
| Line 141: | Line 141: | ||
==See also== |
==See also== |
||
| + | |||
| − | * [[Wine fault]] |
||
== External links == |
== External links == |
||
| Line 155: | Line 155: | ||
[[Category:Aldehydes]] |
[[Category:Aldehydes]] |
||
| + | |||
| − | [[Category:Hazardous air pollutants]] |
||
| − | [[Category:IARC Group 2B carcinogens]] |
||
<!-- |
<!-- |
||
Revision as of 22:48, 6 June 2007
Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
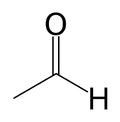
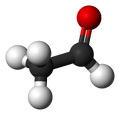
| Acetaldehyde | |
|---|---|
| |
| Common name | acetaldehyde |
| IUPAC name | acetaldehyde |
| Systematic name | ethanal |
| Chemical formula | C2H4O |
| SMILES | CC=O |
| Molecular mass | 44.05 g mol−1 |
| Appearance | Colorless liquid Pungent, fruity odor |
| CAS number | [75-07-0] |
| Properties | |
| Density | 0.788 g cm−3 |
| Solubility in water | soluble in all proportions |
| Melting point | −123.5 °C |
| Boiling point | 20.2 °C |
| Critical temperature | 188 °C at 6.4 MPa |
| Viscosity | ~0.215 at 20 °C |
| Structure | |
| Molecular shape | trigonal planar (sp2) at C1 tetrahedral (sp3) at C2 |
| Dipole moment | 2.7 D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Very flammable (F+) Harmful (Xn) Carc. Cat. 3 |
| NFPA 704 |
|
| R-phrases | R12
, R36/37 , R40
|
| S-phrases |
, S16 , S33 , S36/37 |
| Flash point | −39 °C |
| Autoignition temperature | 185 °C |
| RTECS number | AB1925000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related aldehydes | Formaldehyde Propionaldehyde Ethylene oxide |
| Disclaimer and references | |
Acetaldehyde, sometimes known as ethanal, is an organic chemical compound with the formula CH3CHO or MeCHO. It is a flammable liquid with a fruity smell. Acetaldehyde occurs naturally in ripe fruit, coffee, and fresh bread and is produced by plants as part of their normal metabolism. It is probably best known as the chemical that causes "hangovers".
In the chemical industry, acetaldehyde is used as an intermediate in the production of acetic acid, certain esters, and a number of other chemicals. In 1989, US production stood at 740 million pounds (336,000 t).[How to reference and link to summary or text]
Ethenol
Only a trace of acetaldehyde exists as the enol form, ethenol, with Keq = 6 x 10-5.[1] Ethenol has been detected in the interstellar medium. [How to reference and link to summary or text]
Applications in organic synthesis
Acetaldehyde is a common 2-carbon building block in organic synthesis.[2] Because of its small size and its availability as the anhydrous monomer (unlike formaldehyde), it is a common electrophile. With respect to its condensation reactions, acetaldehyde is prochiral. It is mainly used as a source of the CH3C+H(OH) synthon in aldol and related condensation reactions.[3] Grignard reagents and organolithium compounds react with MeCHO to give hydroxyethyl derivatives.[4] In one of the more spectacular condensation reactions, three equivalents of formaldehyde add to MeCHO to give pentaerythritol, C(CH2OH)4.[5]
In a Strecker reaction, acetaldehyde condenses with cyanide and ammonia to give, after hydrolysis, the amino acid alanine.[6] Acetaldehyde can condense with amines to yield imines, such as the condensation with cyclohexylamine to give N-ethylidenecyclohexylamine. These imines can be used to direct subsequent reactions like an aldol condensation.[7]
It is also an important building block for the synthesis of heterocyclic compounds. A remarkable example is its conversion upon treatment with ammonia to 5-ethyl-2-methylpyridine ("aldehyde-collidine”).[8]
Acetal derivatives
Three molecules of acetaldehyde condense to form “paraldehyde,” a cyclic trimer containing C-O single bonds; four condense to form the cyclic molecule called metaldehyde.
Acetaldehyde forms a stable acetal upon reaction with ethanol under conditions that favor dehydration. The product, CH3CH(OCH2CH3)2, is in fact called "acetal,"[9] although acetal is used more widely to describe other compounds with the formula RCH(OR')2.
Biological aspects
In the liver, the enzyme alcohol dehydrogenase converts ethanol into acetaldehyde, which is then further converted into harmless acetic acid by acetaldehyde dehydrogenase. The last steps of alcoholic fermentation in bacteria, plants and yeast involve the conversion of pyruvate into acetaldehyde by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehydrogenase, now operating in the opposite direction.
Acetaldehyde and hangovers
Most people of East Asian descent have a mutation in their alcohol dehydrogenase gene that makes this enzyme unusually effective at converting ethanol to acetaldehyde, and about half of such people also have a form of acetaldehyde dehydrogenase which is less effective at converting acetaldehyde to acetic acid [10]. This combination causes them to suffer from the alcohol flush reaction, in which acetaldehyde accumulates after drinking, leading to severe and immediate hangover symptoms. These people are therefore less likely to become alcoholics. The drug Antabuse (disulfiram) also prevents the oxidation of acetaldehyde to acetic acid, with the same unpleasant effects for drinkers. It has been used in the treatment of alcoholism.
Other occurrences
Acetaldehyde is an air pollutant resulting from combustion, such as automotive exhaust and tobacco smoke, contributing to the addictive properties of tobacco. [11]
Safety
Acetaldehyde is toxic, an irritant, and a probable carcinogen.[12]
References
- ↑ March, J. “Organic Chemistry: Reactions, Mechanisms, and Structures” J. Wiley, New York: 1992. ISBN 0-471-58148-8.
- ↑ Sowin, T. J.; Melcher, L. M. ”Acetaldehyde” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York.
- REDIRECT Template:Doi
- ↑ Behrens, C.; Paquette, L. A. “N-Benzyl-2,3-Azetidinedione” Organic Syntheses, Collected Volume 10, p.41 (2004)
- ↑ Walter, L. A. “1-(α-Pyridyl)-2-Propanol” Organic Syntheses, Collected Volume 3, p.757 (1955).
- ↑ Schurink, H. B. J. “Pentaerythritol” Organic Syntheses, Collected Volume 1, p.425 (1941). http://www.orgsyn.org/orgsyn/pdfs/CV1P0425.pdf
- ↑ Kendall, E. C. McKenzie, B. F. “dl-Alanine” Organic Syntheses, Collected Volume 1, p.21 (1941). http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf
- ↑ Wittig, G.; Hesse, A. “Directed Aldol Condensations: β-Phenylcinnamaldehyde” Organic Syntheses, Collected Volume 6, p.901 (1988).
- ↑ Frank, R. L.; Pilgrim, F. J.; Riener, E. F. “5-Ethyl-2-Methylpyridine” Organic Syntheses, Collected Volume 4, p. 451 (1963). http://www.orgsyn.org/orgsyn/pdfs/CV4P0451.pdf
- ↑ Adkins, H.; Nissen, B. H. “Acetal” Organic Syntheses, Collected Volume 1, p.1 (1941).http://www.orgsyn.org/orgsyn/pdfs/CV1P0001.pdf
- ↑ Xiao Q, Weiner H, Crabb DW (1996). The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion. J. Clin. Invest. 98 (9): 2027-32.
- ↑ Smoking. (2006). Encyclopædia Britannica. Accessed 27 Oct 2006.
- ↑ http://www.epa.gov/chemfact/s_acetal.txt
See also
External links
- International Chemical Safety Card 0009
- National Pollutant Inventory - Acetaldehde
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph: "Acetaldehyde"
- EPA factsheet about acetaldehyde
- Hal Kibbey, Genetic Influences on Alcohol Drinking and Alcoholism, Indiana University Research and Creative Activity, Vol. 17 no. 3.
- United States Food and Drug Administration (FDA) information for acetaldehyde
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |
